Page 13 - E_Book_Atomic_Theory
P. 13
Chapter Assesment
A. Multiple Choices
1.“ Matter is composed of tiny particles called atoms that cannot be split”. This statement refers
to the atomic theory proposed by …
A. John Dalton
B. J.J. Thomson
C. E. Rutherford
D. Niels Bohr
E. E. Schrodinger
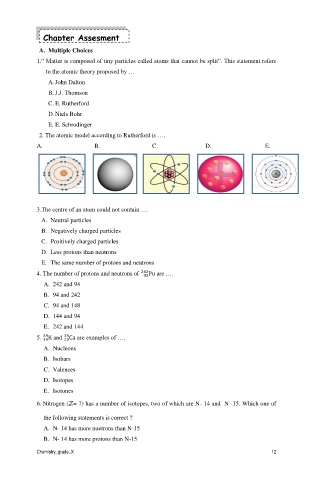
2. The atomic model according to Rutherford is ….
A. B. C. D. E.
3.The centre of an atom could not contain ….
A. Neutral particles
B. Negatively charged particles
C. Positively charged particles
D. Less protons than neutrons
E. The same number of protons and neutrons
4. The number of protons and neutrons of are ….
A. 242 and 94
B. 94 and 242
C. 94 and 148
D. 144 and 94
E. 242 and 144
5. and are examples of ….
A. Nucleons
B. Isobars
C. Valences
D. Isotopes
E. Isotones
6. Nitrogen (Z= 7) has a number of isotopes, two of which are N- 14 and N -15. Which one of
the following statements is correct ?
A. N- 14 has more nuetrons than N-15
B. N- 14 has more protons than N-15
Chemistry_grade_X 12