Page 26 - PCMI Summer Journal 2021
P. 26
The Chemistry and Control of Etching Ferrous Metals with Ferric Chloride Solutions: The Concept of a Constant Etchant Pool Presented by: David M. Allen, Emeritus Professor of Microengineering, Cranfield University, UK
Monitoring the chemical composition of the ferric chloride
Metal dissolution alters the composition of the etchant, reducing [FeCl3] and increasing [FeCl2]. To achieve constant etch rate, it is therefore necessary to control the [FeCl3]/ [FeCl2] ratio. The Nernst equation relates the oxidation-reduction potential (ORP) to this ratio as follows:
E = E0 + 2.303RT log [Fe3+ ] formal nF 10 [Fe2+ ]
where
E = ORP (V)
E0formal = electrode potential in concentrated solutions (V) R = the gas constant (8.31 J/mol K)
T = the absolute temperature (K)
n = the number of electrons changed in the redox reaction F = the Faraday constant (9.6485 x 104 C/mol)
[Fe3+] = the concentration of ferric ions (M)
[Fe2+] = the concentration of ferrous ions (M).
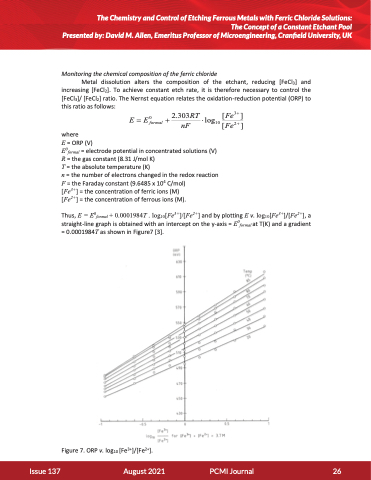
Thus, E = E0formal + 0.0001984T . log10[Fe3+]/[Fe2+] and by plotting E v. log10[Fe3+]/[Fe2+], a straight-line graph is obtained with an intercept on the y-axis = E0formal at T(K) and a gradient = 0.0001984T as shown in Figure7 [3].
Figure 7. ORP v. log10 [Fe3+]/[Fe2+].
Issue 137 August 2021 PCMI Journal 26