Page 24 - PCMI Summer Journal 2021
P. 24
The Chemistry and Control of Etching Ferrous Metals with Ferric Chloride Solutions: The Concept of a Constant Etchant Pool Presented by: David M. Allen, Emeritus Professor of Microengineering, Cranfield University, UK
Control of free hydrochloric acid in the etchant
As stated previously, to manually titrate free HCl with a base, the FeCl3 must be complexed first to prevent hydrolysis and liberation of additional HCl not present in the original etchant formulation. Typical titration processes are shown in Table 3. Such processes are not easy to perform in used etchants, take time to process and are off-line so that results are not immediately available for monitoring and control adjustment.
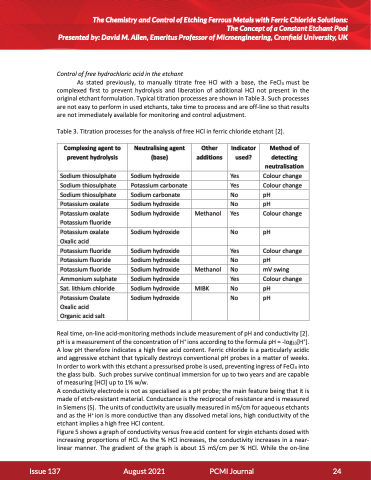
Table 3. Titration processes for the analysis of free HCl in ferric chloride etchant [2].
Complexing agent to prevent hydrolysis
Neutralising agent (base)
Other additions
Indicator used?
Method of detecting neutralisation
Sodium thiosulphate Sodium thiosulphate Sodium thiosulphate Potassium oxalate Potassium oxalate Potassium fluoride Potassium oxalate Oxalic acid Potassium fluoride Potassium fluoride Potassium fluoride Ammonium sulphate Sat. lithium chloride
Sodium hydroxide Potassium carbonate Sodium carbonate Sodium hydroxide Sodium hydroxide
Sodium hydroxide
Sodium hydroxide Sodium hydroxide Sodium hydroxide Sodium hydroxide Sodium hydroxide
Methanol
Methanol MIBK
Yes Colour change Yes Colour change No pH
No pH
Yes Colour change No pH
Yes Colour change No pH
No mV swing
Yes Colour change No pH
Potassium Oxalate Oxalic acid Organic acid salt
Sodium hydroxide
No
pH
Real time, on-line acid-monitoring methods include measurement of pH and conductivity [2]. pH is a measurement of the concentration of H+ ions according to the formula pH = -log10[H+]. A low pH therefore indicates a high free acid content. Ferric chloride is a particularly acidic and aggressive etchant that typically destroys conventional pH probes in a matter of weeks. In order to work with this etchant a pressurised probe is used, preventing ingress of FeCl3 into the glass bulb. Such probes survive continual immersion for up to two years and are capable of measuring [HCl] up to 1% w/w.
A conductivity electrode is not as specialised as a pH probe; the main feature being that it is made of etch-resistant material. Conductance is the reciprocal of resistance and is measured in Siemens (S). The units of conductivity are usually measured in mS/cm for aqueous etchants and as the H+ ion is more conductive than any dissolved metal ions, high conductivity of the etchant implies a high free HCl content.
Figure 5 shows a graph of conductivity versus free acid content for virgin etchants dosed with increasing proportions of HCl. As the % HCl increases, the conductivity increases in a near- linear manner. The gradient of the graph is about 15 mS/cm per % HCl. While the on-line
Issue 137 August 2021 PCMI Journal 24