Page 32 - PCMI Summer Journal 2021
P. 32
An Overview of the Operation of Ferric Chloride Regeneration Systems in Photochemical Etching Machines
Presented by: Kirk Lauver, the Marketing Specialist, Chemcut Corporation, US
7/27/21
2
3
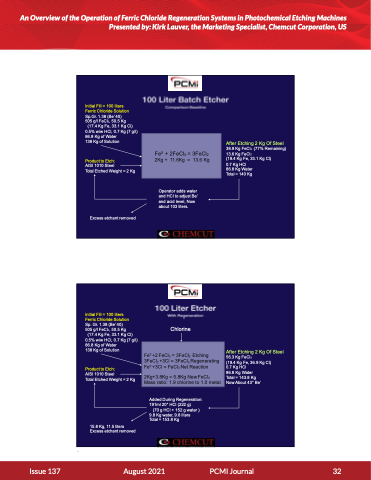
Initial Fill = 100 liters Ferric Chloride Solution Sp.Gr. 1.38 (Be’ 40) 505 g/l FeCl3, 50.5 Kg
(17.4 Kg Fe, 33.1 Kg Cl) 0.5% w/w HCl, 0.7 Kg (7 g/l) 86.8 Kg of Water
138 Kg of Solution
Product to Etch:
AISI 1010 Steel
Total Etched Weight = 2 Kg
Excess etchant removed
After Etching 2 Kg Of Steel
38.9 Kg FeCl3 (77% Remaining) 13.6 Kg FeCl2
(19.4 Kg Fe, 33.1 Kg Cl)
0.7 Kg HCl
86.8 Kg Water Total = 140 Kg
Fe0 + 2FeCl3 = 3FeCl2 2Kg + 11.6Kg = 13.6 Kg
Operator adds water and HCl to adjust Be’ and acid level, Now about 103 liters.
Initial Fill = 100 liters Ferric Chloride Solution Sp. Gr. 1.38 (Be’ 40) 505 g/l FeCl3, 50.5 Kg
(17.4 Kg Fe, 33.1 Kg Cl) 0.5% w/w HCl, 0.7 Kg (7 g/l) 86.8 Kg of Water
138 Kg of Solution
Product to Etch:
AISI 1010 Steel
Total Etched Weight = 2 Kg
15.8 Kg, 11.5 liters Excess etchant removed
Chlorine
Added During Regeneration: 191ml 20° HCl (222 g)
(70 g HCl + 152 g water ) 9.8 Kg water, 9.8 liters Total = 153.8 Kg
After Etching 2 Kg Of Steel
56.3 Kg FeCl3
(19.4 Kg Fe, 36.9 Kg Cl) 0.7 Kg HCl
86.8 Kg Water
Total = 143.8 Kg
Now About 43° Be’
Fe0 +2 FeCl3 = 3FeCl2 Etching 3FeCl2 +3Cl = 3FeCl3 Regenerating Fe0 +3Cl = FeCl3 Net Reaction
2Kg+3.8Kg = 5.8Kg New FeCl3 Mass ratio: 1.9 chlorine to 1.0 metal
Issue 137 August 2021 PCMI Journal 32