Page 22 - Lecture 3
P. 22
Dispersion Forces
• All molecules experience these forces.
• At any given instant there may be more electrons at
one end of a molecule than at the other, giving a short-
lived polarity.
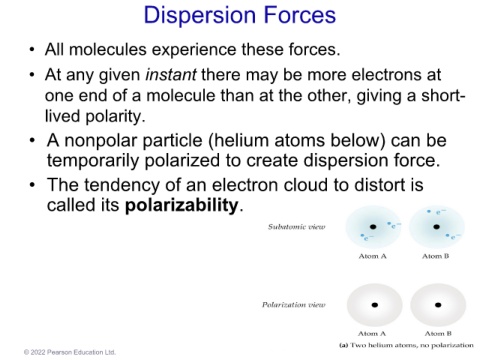
• A nonpolar particle (helium atoms below) can be
temporarily polarized to create dispersion force.
• The tendency of an electron cloud to distort is
called its polarizability.
Basic Concepts
of Chemical
Bonding
© 2022 Pearson Education Ltd.