Page 8 - Lecture 3
P. 8
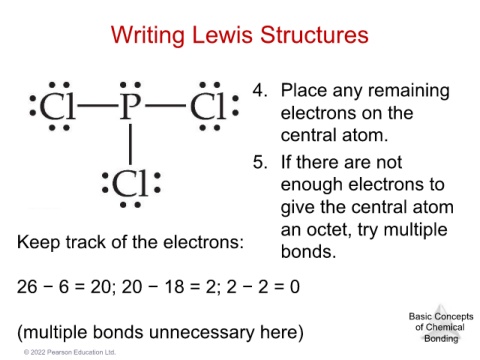
Writing Lewis Structures
4. Place any remaining
electrons on the
central atom.
5. If there are not
enough electrons to
give the central atom
Keep track of the electrons: an octet, try multiple
bonds.
26 − 6 = 20; 20 − 18 = 2; 2 − 2 = 0
(multiple bonds unnecessary here) Basic Concepts
of Chemical
© 2022 Pearson Education Ltd.
Bonding