Page 52 - E-Modul ASAM DAN BASA
P. 52
KIMIA XI
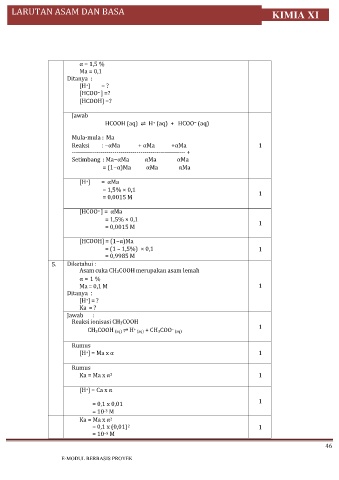
α = 1,5 %
Ma = 0,1
Ditanya :
[H ] = ?
+
[HCOO ] =?
−
[HCOOH] =?
Jawab
HCOOH (aq) ⇌ H (aq) + HCOO (aq)
+
−
Mula-mula : Ma
Reaksi : −αMa + αMa +αMa 1
-------------------------------------------------------- +
Setimbang : Ma−αMa αMa αMa
= (1−α)Ma αMa αMa
[H ] = αMa
+
= 1,5% × 0,1
= 0,0015 M 1
[HCOO ] = αMa
−
= 1,5% × 0,1 1
= 0,0015 M
[HCOOH] = (1−α)Ma
= (1 – 1,5%) × 0,1 1
= 0,9985 M
5. Diketahui :
Asam cuka CH3COOH merupakan asam lemah
α = 1 %
Ma = 0,1 M 1
Ditanya :
+
[H ] = ?
Ka = ?
Jawab :
Reaksi ionisasi CH3COOH
+ – 1
CH3COOH (aq) ⇌ H (aq) + CH3COO (aq)
Rumus
[H ] = Ma x α 1
+
Rumus
2
Ka = Ma x α 1
+
[H ] = Ca x α
1
= 0,1 x 0,01
= 10 M
-3
Ka = Ma x α
2
2
= 0,1 x (0,01) 1
= 10 M
–5
46
E-MODUL BERBASIS PROYEK