Page 140 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 140
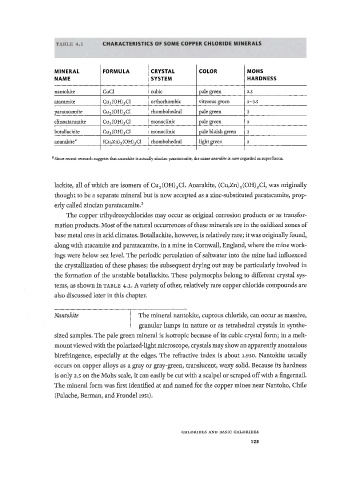
TABLE 4.1 CHARACTERISTICS OF SOME COPPER CHLORIDE MINERALS
MINERAL FORMULA CRYSTAL COLOR MOHS
NAME SYSTEM HARDNESS
nantokite CuCl cubic pale green 2.5
atacamite Cu 2 (OH) 3 Cl orthorhombic vitreous green 3-3.5
paratacamite Cu 2 (OH) 3 Cl rhombohedral pale green 3
clinoatacamite Cu 2 (OH) 3 Cl monoclinic pale green 3
botallackite Cu 2 (OH) 3 Cl monoclinic pale bluish green 3
anarakite 3 (Cu,Zn) 2(OH) 3Cl rhombohedral light green 3
a Since recent research suggests that anarakite is actually zincian paratacamite, the name anarakite is now regarded as superfluous.
lackite, all of which are isomers of Cu 2 (OH) 3 Cl. Anarakite, (Cu,Zn) 2 (OH) 3 Cl, was originally
thought to be a separate mineral but is now accepted as a zinc-substituted paratacamite, prop
erly called zincian paratacamite. 2
The copper trihydroxychlorides may occur as original corrosion products or as transfor
mation products. Most of the natural occurrences of these minerals are in the oxidized zones of
base metal ores in arid climates. Botallackite, however, is relatively rare; it was originally found,
along with atacamite and paratacamite, in a mine in Cornwall, England, where the mine work
ings were below sea level. The periodic percolation of saltwater into the mine had influenced
the crystallization of these phases; the subsequent drying out may be particularly involved in
the formation of the unstable botallackite. These polymorphs belong to different crystal sys
tems, as shown in TABLE 4.1. A variety of other, relatively rare copper chloride compounds are
also discussed later in this chapter.
Nantokite The mineral nantokite, cuprous chloride, can occur as massive,
granular lumps in nature or as tetrahedral crystals in synthe
sized samples. The pale green mineral is isotropic because of its cubic crystal form; in a melt-
mount viewed with the polarized-light microscope, crystals may show an apparendy anomalous
birefringence, especially at the edges. The refractive index is about 1.930. Nantokite usually
occurs on copper alloys as a gray or gray-green, translucent, waxy solid. Because its hardness
is only 2.5 on the Mohs scale, it can easily be cut with a scalpel or scraped off with a fingernail.
The mineral form was first identified at and named for the copper mines near Nantoko, Chile
(Palache, Berman, and Frondel i95i).
C H L O R I D E S AN D BASI C C H L O R I D E S
123