Page 152 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 152
TABLE 4.2 CHLORIDE ION CONCENTRATIONS AND EQUILIBRIA"
CHLORIDE SIMILAR C u 2 + + Cu +4CI" = 2CuCI 2 C u 2 + + Cu + 2CI" = 2CuCI
CONCENTRATION AQUEOUS Cu + 2CI" = CuCl" + e" Cu + CP = CuCl + e"
2
IN PPM ENVIRONMENT Cu 2+ + 2CI" + e" = CuCI 2- Cu 2+ + Cl~ + e~ = CuCl
1 deionized water + 75 + 12
100 Perth tap water + 29 - 1 1
10,000 dilute seawater -1 7 - 3 4
100,000 pit solution - 3 9 - 4 6
After MacLeod (i98i).
All changes in Gibbs free energy are shown here in kcal/mol.
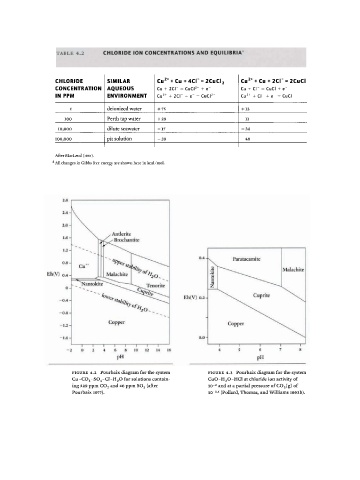
F I G U R E 4. 2 Pourbaix diagram for the system F I G U R E 4.3 Pourbaix diagram for the system
Cu-C0 3 -S0 4 -Cl-H 2 0 for solutions contain CuO-H 2 0-HCl at chloride ion activity of
4
ing 229 ppm C0 2 and 6 ppm S0 3 (after io - 2 and at a partial pressure of C0 2(g) of
τ» 1 , \ ίο · 5 (Pollard, Thomas, and Williams 1992b).
-3