Page 46 - MEMENTO THERAPEUTIQUE RCP 2024
P. 46
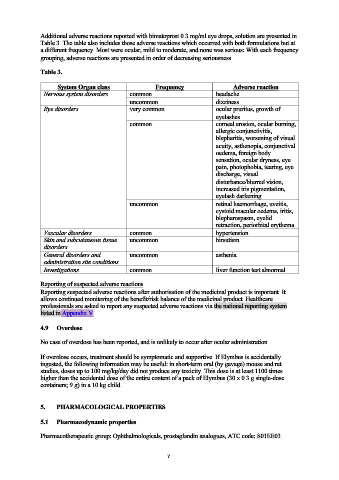
Additional adverse reactions reported with bimatoprost 0.3 mg/ml eye drops, solution are presented in
Table 3. The table also includes those adverse reactions which occurred with both formulations but at
a different frequency. Most were ocular, mild to moderate, and none was serious: With each frequency
grouping, adverse reactions are presented in order of decreasing seriousness.
Table 3.
System Organ class Frequency Adverse reaction
Nervous system disorders common headache
uncommon dizziness
Eye disorders very common ocular pruritus, growth of
eyelashes
common corneal erosion, ocular burning,
allergic conjunctivitis,
blepharitis, worsening of visual
acuity, asthenopia, conjunctival
oedema, foreign body
sensation, ocular dryness, eye
pain, photophobia, tearing, eye
discharge, visual
disturbance/blurred vision,
increased iris pigmentation,
eyelash darkening
uncommon retinal haemorrhage, uveitis,
cystoid macular oedema, iritis,
blepharospasm, eyelid
retraction, periorbital erythema
Vascular disorders common hypertension
Skin and subcutaneous tissue uncommon hirsutism
disorders
General disorders and uncommon asthenia
administration site conditions
Investigations common liver function test abnormal
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It
allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare
professionals are asked to report any suspected adverse reactions via the national reporting system
listed in Appendix V.
4.9 Overdose
No case of overdose has been reported, and is unlikely to occur after ocular administration.
If overdose occurs, treatment should be symptomatic and supportive. If Elymbus is accidentally
ingested, the following information may be useful: in short-term oral (by gavage) mouse and rat
studies, doses up to 100 mg/kg/day did not produce any toxicity. This dose is at least 1100 times
higher than the accidental dose of the entire content of a pack of Elymbus (30 x 0.3 g single-dose
containers; 9 g) in a 10 kg child.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, prostaglandin analogues, ATC code: S01EE03.
7