Page 91 - MEMENTO THERAPEUTIQUE RCP 2024
P. 91
MONOPROST MD_FR/H/0499/001-002/IA/036
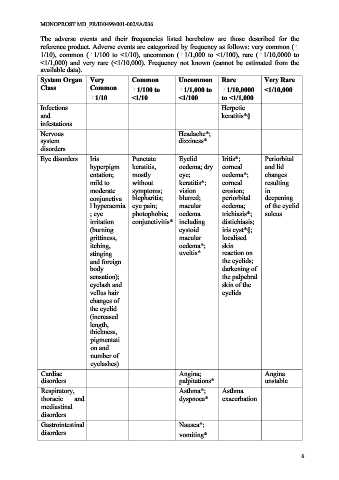
The adverse events and their frequencies listed herebelow are those described for the
reference product. Adverse events are categorized by frequency as follows: very common (
1/10), common ( 1/100 to <1/10), uncommon ( 1/1,000 to <1/100), rare ( 1/10,0000 to
<1/1,000) and very rare (<1/10,000). Frequency not known (cannot be estimated from the
available data).
System Organ Very Common Uncommon Rare Very Rare
Class Common 1/100 to 1/1,000 to 1/10,0000 <1/10,000
1/10 <1/10 <1/100 to <1/1,000
Infections Herpetic
and keratitis*§
infestations
Nervous Headache*;
system dizziness*
disorders
Eye disorders Iris Punctate Eyelid Iritis*; Periorbital
hyperpigm keratitis, oedema; dry corneal and lid
entation; mostly eye; oedema*; changes
mild to without keratitis*; corneal resulting
moderate symptoms; vision erosion; in
conjunctiva blepharitis; blurred; periorbital deepening
l hyperaemia eye pain; macular oedema; of the eyelid
; eye photophobia; oedema trichiasis*; sulcus
irritation conjunctivitis* including distichiasis;
(burning cystoid iris cyst*§;
grittiness, macular localised
itching, oedema*; skin
stinging uveitis* reaction on
and foreign the eyelids;
body darkening of
sensation); the palpebral
eyelash and skin of the
vellus hair eyelids
changes of
the eyelid
(increased
length,
thickness,
pigmentati
on and
number of
eyelashes)
Cardiac Angina; Angina
disorders palpitations* unstable
Respiratory, Asthma*; Asthma
thoracic and dyspnoea* exacerbation
mediastinal
disorders
Gastrointestinal Nausea*;
disorders vomiting*
6