Page 29 - NATASYA E-MODUL larutan Penyangga
P. 29
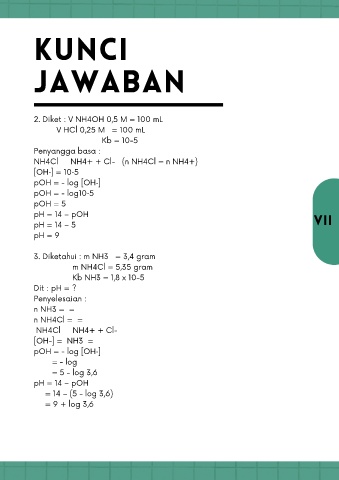
KUNCI
JAWABAN
2. Diket : V NH4OH 0,5 M = 100 mL
V HCl 0,25 M = 100 mL
Kb = 10-5
Penyangga basa :
NH4Cl → NH4+ + Cl- (n NH4Cl = n NH4+)
[OH‑] = 10‑5
pOH = - log [OH‑]
pOH = - log10‑5
pOH = 5
pH = 14 – pOH
pH = 14 – 5 VII
pH = 9
3. Diketahui : m NH3 = 3,4 gram
m NH4Cl = 5,35 gram
Kb NH3 = 1,8 x 10-5
Dit : pH = ?
Penyelesaian :
n NH3 = =
n NH4Cl = =
NH4Cl → NH4+ + Cl-
[OH-] = NH3 =
pOH = - log [OH‑]
= - log
= 5 - log 3,6
pH = 14 – pOH
= 14 – (5 - log 3,6)
= 9 + log 3,6