Page 85 - Practical-workbook-organic-3 isa
P. 85
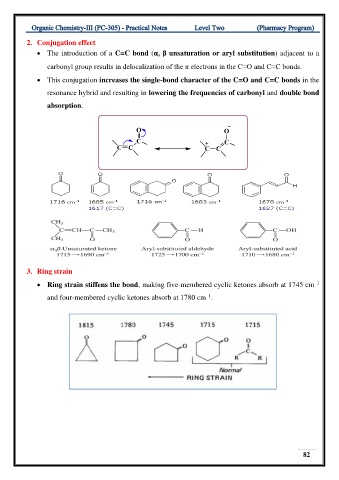
2. Conjugation effect
• The introduction of a C=C bond (α, β unsaturation or aryl substitution) adjacent to a
carbonyl group results in delocalization of the π electrons in the C=O and C=C bonds.
• This conjugation increases the single-bond character of the C=O and C=C bonds in the
resonance hybrid and resulting in lowering the frequencies of carbonyl and double bond
absorption.
3. Ring strain
–1
• Ring strain stiffens the bond, making five-membered cyclic ketones absorb at 1745 cm
–1
and four-membered cyclic ketones absorb at 1780 cm .
82