Page 130 - SSAB Welding Handbook Edition 2
P. 130
©2009-2019 by SSAB Group of companies (SSAB). All rights reserved. Only digital PDF file. No distribution. No printing allowed!
No part of this handbook may be reproduced in any form or by any means without permission in writing from SSAB.
22.0 Hardening mechanisms in the heat-affected zone and the unaffected parent metal Welding handbook
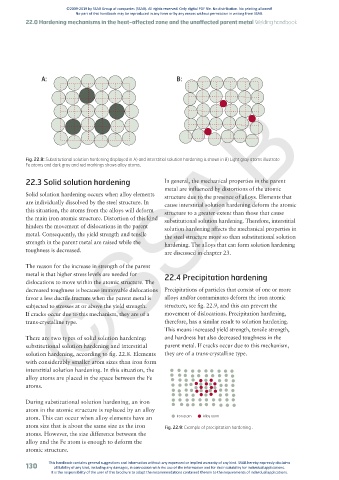
A: B:
©SSAB
Fig. 22.8: Substitutional solution hardening displayed in A) and interstitial solution hardening is shown in B) Light gray atoms illustrate
Fe atoms and dark gray and red markings shows alloy atoms.
22.3 Solid solution hardening In general, the mechanical properties in the parent
metal are influenced by distortions of the atomic
Solid solution hardening occurs when alloy elements structure due to the presence of alloys. Elements that
are individually dissolved by the steel structure. In cause interstitial solution hardening deform the atomic
this situation, the atoms from the alloys will deform structure to a greater extent than those that cause
the main iron atomic structure. Distortion of this kind substitutional solution hardening. Therefore, interstitial
hinders the movement of dislocations in the parent solution hardening affects the mechanical properties in
metal. Consequently, the yield strength and tensile the steel structure more so than substitutional solution
strength in the parent metal are raised while the hardening. The alloys that can form solution hardening
toughness is decreased. are discussed in chapter 23.
The reason for the increase in strength of the parent
metal is that higher stress levels are needed for 22.4 Precipitation hardening
dislocations to move within the atomic structure. The
decreased toughness is because immovable dislocations Precipitations of particles that consist of one or more
favor a less ductile fracture when the parent metal is alloys and/or contaminates deform the iron atomic
subjected to stresses at or above the yield strength. structure, see fig. 22.9, and this can prevent the
If cracks occur due to this mechanism, they are of a movement of dislocations. Precipitation hardening,
trans-crystalline type. therefore, has a similar result to solution hardening.
This means increased yield strength, tensile strength,
There are two types of solid solution hardening: and hardness but also decreased toughness in the
substitutional solution hardening and interstitial parent metal. If cracks occur due to this mechanism,
solution hardening, according to fig. 22.8. Elements they are of a trans-crystalline type.
with considerably smaller atom sizes than iron form
interstitial solution hardening. In this situation, the
alloy atoms are placed in the space between the Fe
atoms.
During substitutional solution hardening, an iron
atom in the atomic structure is replaced by an alloy
atom. This can occur when alloy elements have an Iron atom Alloy atom
atom size that is about the same size as the iron Fig. 22.9: Example of precipitation hardening .
atoms. However, the size difference between the
alloy and the Fe atom is enough to deform the
atomic structure.
130 This handbook contains general suggestions and information without any expressed or implied warranty of any kind. SSAB hereby expressly disclaims
all liability of any kind, including any damages, in connection with the use of the information and for their suitability for individual applications.
It is the responsibility of the user of this brochure to adapt the recommendations contained therein to the requirements of individual applications.