Page 62 - PC 101 practical notes 24-25..
P. 62
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
2) The concentration of borax can be calculated from either E.P 1 or E.P 2.
3) Borax is considered a sodium salt of boric acid and the reaction between
HCl & borax is an example of displacement titration where HCl (strong
acid) displaces boric acid (very weak acid) in its salts.
Na 2B 4O 7 + 7 H 2O → 2 NaOH + 4 H 3BO 3
2 NaOH + 2 HCl → 2 NaCl + 2 H 2O
.
by summation: Na 2B 4O 7 + 5 H 2O + 2 HCl → 4 H 3BO 3 + 2 NaCl
Important Question :
As we discussed before, borax can be determined through its NaOH
content by titration against 0.1 N HCl (E.P 1) and then it can be again
determined through its boric acid content by adding glycerol to the above
titrated solution and then titration against 0.1 N NaOH (E.P 2).
Q1- What is the relation between E.P 1 and E.P 2 ?
Answer: E.P 2 = 2 E.P 1 ............... as mentioned before
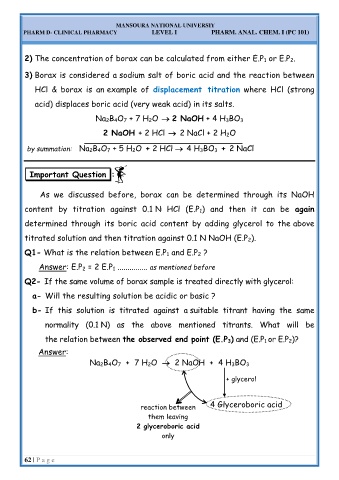
Q2- If the same volume of borax sample is treated directly with glycerol:
a- Will the resulting solution be acidic or basic ?
b- If this solution is titrated against a suitable titrant having the same
normality (0.1 N) as the above mentioned titrants. What will be
the relation between the observed end point (E.P 3) and (E.P 1 or E.P 2)?
Answer:
Na 2B 4O 7 + 7 H 2O → 2 NaOH + 4 H 3BO 3
+ glycerol
reaction between 4 Glyceroboric acid
them leaving
2 glyceroboric acid
only
62 | P a g e