Page 12 - PowerPoint Presentation
P. 12
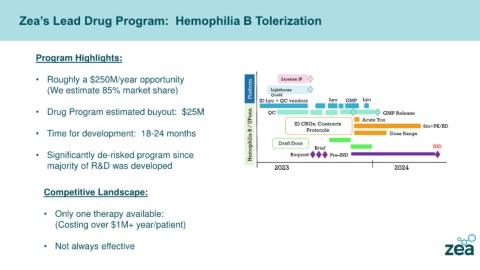
Zea’s Lead Drug Program: Hemophilia B Tolerization
Program Highlights:
• Roughly a $250M/year opportunity License IP
(We estimate 85% market share) Platform Lighthouse
Qualif.
ID Lyo + QC vendors Lyo GMP Lyo
• Drug Program estimated buyout: $25M QC Acute Tox
Hemophilia B / UPenn Draft Docs
GMP Release
Protocols
• Time for development: 18-24 months ID CROs; Contracts Dose Range 6m+PK/BD
• Significantly de-risked program since Request Brief Pre-IND IND
majority of R&D was developed 2023 2024
Competitive Landscape:
• Only one therapy available:
(Costing over $1M+ year/patient)
• Not always effective