Page 26 - E-LKM ACID BASE_Neat
P. 26
Lembar Kerja Mahasiswa - Asam Basa
3. Polyprotic Acids
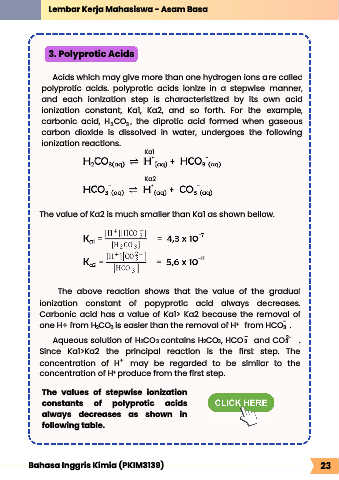
Acids which may give more than one hydrogen ions are called
polyprotic acids. polyprotic acids ionize in a stepwise manner,
and each ionization step is characteristized by its own acid
ionization constant, Ka1, Ka2, and so forth. For the example,
carbonic acid, H CO , the diprotic acid formed when gaseous
3
2
carbon dioxide is dissolved in water, undergoes the following
ionization reactions.
Ka1
Ka2
The value of Ka2 is much smaller than Ka1 as shown bellow.
The above reaction shows that the value of the gradual
ionization constant of popyprotic acid always decreases.
Carbonic acid has a value of Ka1> Ka2 because the removal of
-
one H+ from H CO is easier than the removal of H from HCO .
+
2
3
3
_ 2-
Aqueous solution of H CO contains H CO , HCO and CO .
2
3
2
3
3
3
Since Ka1>Ka2 the principal reaction is the first step. The
+
concentration of H may be regarded to be similar to the
+
concentration of H produce from the first step.
The values of stepwise ionization
constants of polyprotic acids
always decreases as shown in
following table.
Bahasa Inggris Kimia (PKIM3139) 23