Page 27 - DIFDUF
P. 27
697
1.56 ± 1.14
2
30.3 ± 6.0
The frequency of NOD2/CARD15 mutations is relatively
8.74 ± 1.97
72 (47.1%)
81 (52.9%)
67.5 ± 13.06
n=153
Interestingly, in our cohort only one of the three NOD2/
Total
As expected, and in accordance with previous findings, we
1.56 ± 1.14
30.3 ± 6.0
2
8.74 ± 1.97
72 (47%)
81 (53%)
67.5 ± 13.06
n=153
0.23
Total
0.65
0.32
0.54
prescribed.
0.61
been prescribed, and “no adherence” if no insulin had been
0.54
insulin, “partial adherence” if only one kind of insulin had
0.43
if the primary physician had prescribed basal and bolus
0.63
Adherence by physicians was defined as “full adherence”
0.86
pHySICIAn ADHEREnCE
0.45
0.61
adherence” if the patient did not purchase any insulin.
purchased only one kind of insulin (basal or bolus), and “no
0.47
(short-acting) insulin, “partial adherence” if the patient had
0.08
patient had purchased basal (long-acting) as well as bolus
0.005
Adherence by patients was defined as “full adherence” if the
0.04
pATIEnT ADHEREnCE
0.07
value
Center.
P
by the research ethics committee at Assaf Harofeh Medical
from 3–6 months before admission. Our study was approved
each patient before admission was analyzed. The results were
hospital electronic database. The last available HbA1c of
The Gly908Arg mutation carriers were younger in age
patient database, as this information is not available in the
purchasing of insulin were retrieved from the Israeli national
first month post-discharge data regarding prescription and
Pre-hospitalization hemoglobin A1C (HbA1c) levels and
pitalization.
were based on the clinical judgment of physicians during hos-
hospital electronic database. The discharge recommendations
REVIEWS 1 (5.9) 4 (24) 12 (71) 1 (5.9) 8 (47) 1 (5.9) 7 (41) 6 (5–15) 7 (41.2) 6 (37.5) 6 (35.3) 4 (23.5) 5 (33.3) 7 (41.2) 28.82 ± 9.1 38.88 ± 10.4 n=17 (68%) Non-carrier 0.24 0.41 0.50 0.75 – 0.49 0.88 – 0.53 0.06 0.006 value P Original articles 9 (41.1) 9 (40.9) 4 (18.2) 2 (10.0) 0 5 (22.7) 1 (4.5) 0 11 (50.0) 30.7 ± 12.7 41.2 ± 12.3 n=22 (88%) Non-carrier 2 (25) 2 (25) 4 (50) 1 (12.5) 4 (50) 1 (12.5) 2 (25)
#
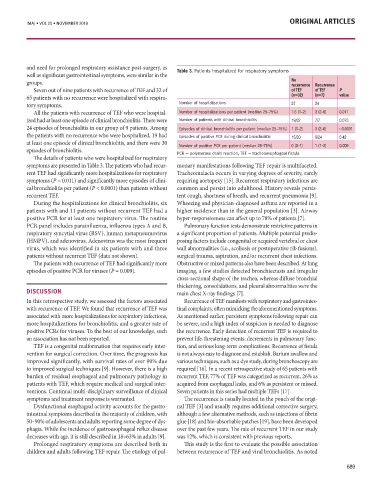
Comparison of the Clinical utility in the Detection of and need for prolonged respiratory assistance post-surgery, as Table 3. Patients hospitalized for respiratory symptoms
Anti-nuclear Antibodies Between the Elia CTD Screen well as significant gastrointestinal symptoms, were similar in the No
groups.
recurrence Recurrence
Seven out of nine patients with recurrence of TEF and 32 of
and Indirect Immunofluorescence on Hep-2 Cells: 65 patients with no recurrence were hospitalized with respira- Number of hospitalizations of TEF of TEF P
value
(n=7)
(n=32)
57
34
A Review of the literature tory symptoms. Number of hospitalizations per patient (median 25–75%) 1.5 (1–2) 3 (2–6) 0.011
All the patients with recurrence of TEF who were hospital-
ized had at least one episode of clinical bronchiolitis. There were Number of patients with clinical bronchiolitis 19/32 7/7 0.073
Christoph Robier MD 1,4 Omid Amouzadeh-Ghadikolai MD , Mariana Stettin MD and Gerhard Reicht MD 2 24 episodes of bronchiolitis in our group of 9 patients. Among Episodes of clinical bronchiolitis per patient (median 25–75%) 1 (1–2) 3 (2–6) < 0.0001
1
3
the patients with no recurrence who were hospitalized, 19 had Episodes of positive PCR during clinical bronchiolitis
3
1 Institute of Laboratory Diagnostics and Departments of Internal Medicine and Psychiatry, Hospital of the Brothers of St. John of God, Graz, Austria at least one episode of clinical bronchiolitis, and there were 30 15/30 9/24 0.42
2
4 Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria Number of positive PCR per patient (median 25–75%) 0 (0–1) 1 (1–2) 0.009
episodes of bronchiolitis. PCR = polymerase chain reaction, TEF = tracheoesophageal fistula
131118-COHANIM - 131118-COHANIM | 4 - B | 18-11-13 | 11:24:13 | SR:-- | Magenta
The details of patients who were hospitalized for respiratory
131118-COHANIM - 131118-COHANIM | 4 - B | 18-11-13 | 11:24:13 | SR:-- | Yellow
#131118-COHANIM - 131118-COHANIM | 4 - B | 18-11-13 | 11:24:13 | SR:-- | Black
symptoms are presented in Table 3. The patients who had recur- monary manifestations following TEF repair is multifaceted.
131118-COHANIM - 131118-COHANIM | 4 - B | 18-11-13 | 11:24:13 | SR:-- | Cyan
rent TEF had significantly more hospitalizations for respiratory Tracheomalacia occurs in varying degrees of severity, rarely
clinical suspicion is strong and the alternative method is nega- symptoms (P = 0.011) and significantly more episodes of clini- requiring aortopexy [15]. Recurrent respiratory infections are
KEY WORDS: antinuclear antibodies (ANA), Elia CTD Screen (ECS), tive, it is mandatory to perform IIF [1]. Because both IIF and cal bronchiolitis per patient (P < 0.0001) than patients without common and persist into adulthood. History reveals persis-
solid-phase assay solid-phase assays have positive properties, in addition to weak recurrent TEF. tent cough, shortness of breath, and recurrent pneumonia [9].
IMAJ 2018; 20: 700–702 points, a combination of the two techniques may improve the During the hospitalizations for clinical bronchiolitis, six Wheezing and physician-diagnosed asthma are reported in a
screening procedure for ANA. patients with and 11 patients without recurrent TEF had a higher incidence than in the general population [3]. Airway
In this review, we discuss studies that have evaluated the Elia positive PCR for at least one respiratory virus. The routine hyper-responsiveness can affect up to 78% of patients [7].
CTD Screen (ECS) assay (Phadia, Thermo Fisher Scientific, PCR panel includes parainfluenza, influenza types A and B, Pulmonary function tests demonstrate restrictive patterns in
he detection of antinuclear antibodies (ANA) is important Freiburg, Germany) to determine the optimal role of this test respiratory syncytial virus (RSV), human metapneumovirus a significant proportion of patients. Multiple potential predis-
T in the diagnosis of systemic autoimmune disorders such as in the diagnostic process. (HMPV), and adenovirus. Adenovirus was the most frequent posing factors include congenital or acquired vertebral or chest
systemic lupus erythematosus (SLE), systemic sclerosis (SSc), virus, which was identified in six patients with and three wall abnormalities (i.e., scoliosis or postoperative rib fusions),
Sjögren’s syndrome, mixed connective tissue disease (MCTD), ECS ASSAy patients without recurrent TEF (data not shown). surgical trauma, aspiration, and/or recurrent chest infections.
and polymyositis. ANA are thus incorporated in several clas- The ECS is a solid-phase fluoroenzymeimmunoassay. Each well The patients with recurrence of TEF had significantly more Obstructive or mixed patterns also have been described. At lung
sification criteria [1,2]. Beyond that, there is an open discus- of the ECS is coated with the following 17 antigens: dsDNA, SSA/ episodes of positive PCR for viruses (P = 0.009). imaging, a few studies detected bronchiectasis and irregular
sion whether ANA represent useful biomarkers in preventive Ro 52, SSA/Ro 60, SSB/La, U1-RNP (RNP70, A, C), Sm, CENP-B, cross-sectional shape of the trachea, whereas diffuse bronchial
medicine [3], especially for the identification of pre- or early Jo-1, Scl-70, Rib-P, fibrillarin, RNA polymerase III, PM-Scl, PCNA, thickening, consolidations, and pleural abnormalities were the
disease with an “intent to prevent” Immunofluorescence on Hep-2 cells and and Mi-2. All of the antigens that are DISCUSSION main chest X-ray findings [7].
approach [2,4]. included, except for dsDNA which In this retrospective study, we assessed the factors associated Recurrence of TEF manifests with respiratory and gastrointes-
Indirect immunofluorescence Elia connective tissue disease screening is a native purified antigen, are with recurrence of TEF. We found that recurrence of TEF was tinal complaints, often mimicking the aforementioned symptoms.
(IIF) on Hep-2 cells is regarded as assay differ significantly in the human recombinant proteins. The associated with more hospitalizations for respiratory infections, As mentioned earlier, persistent symptoms following repair can
the gold standard for ANA screen- sensitivity and specificity for antinuclear cut-off levels for all antibody results, more hospitalizations for bronchiolitis, and a greater rate of be severe, and a high index of suspicion is needed to diagnose
ing and has been defined as the antibodies-associated disorders with the exception of U1RNP, are: positive PCRs for viruses. To the best of our knowledge, such the recurrence. Early detection of recurrent TEF is required to
reference screening method for ANA in the clinical laboratory negative < 7 U/ml, equivocal 7–10 U/ml, and positive > 10 U/ml. an association has not been reported. prevent life-threatening events, decrements in pulmonary func-
routine [1]. However, it has been shown that some subtypes of Cut-off for U1RNP antibody results are: negative < 5 U/ml, equivo- TEF is a congenital malformation that requires early inter- tion, and serious long-term complications. Recurrence of fistula
ANA, especially anti-SSA/Ro and anti-Jo-1 antibodies, may be cal 5–10 U/ml, equivocal, and positive > 10 U/ml [8]. vention for surgical correction. Over time, the prognosis has is not always easy to diagnose and establish. Barium swallow and
overlooked by IIF [5]. Furthermore, IIF requires experienced and improved significantly, with survival rates of over 90% due various techniques, such as a dye study, during bronchoscopy are
well-trained analysts, is quite time-consuming, and shows high COnCORDAnCE Of THE ECS wITH IIf to improved surgical techniques [9]. However, there is a high required [16]. In a recent retrospective study of 65 patients with
inter-observer variability [6]. To overcome these disadvantages, In some published studies, the concordance between the ECS burden of residual esophageal and pulmonary pathology in recurrent TEF, 77% of TEF was categorized as recurrent, 26% as
commercially available automated solid-phase assays incorporat- and IIF has been determined. The concordance is calculated by patients with TEF, which require medical and surgical inter- acquired from esophageal leaks, and 6% as persistent or missed.
ing a mixture of various extracted antigens have been developed. the sum of all ECS positive /IIF positive and ECS negative /IIF negative study ventions. Continual multi-disciplinary surveillance of clinical Seven patients in this series had multiple TEFs [17].
For laboratories with a high throughput of samples, such auto- subjects. The available studies showed similar results, report- symptoms and treatment response is warranted. The recurrence is usually located in the pouch of the origi-
mated tests may be beneficial. The diagnostic potential of auto- ing concordance rates of 78.8% [9], 79.2% [10], and 83.1% [8], Dysfunctional esophageal activity accounts for the gastro- nal TEF [3] and usually requires additional corrective surgery,
mated assays is restricted to the antigens incorporated in the panel respectively. intestinal symptoms described in the majority of children, with although a few alternative methods, such as injections of fibrin
of the test, which is a clear limitation for the use of solid-phase DIffEREnCES In THE DETECTIOn Of AnA BETwEEn THE ECS AnD IIf 50–90% of adolescents and adults reporting some degree of dys- glue [18] and bio-absorbable patches [19], have been developed
phagia. While the incidence of gastroesophageal reflux disease
over the past few years. The rate of recurrent TEF in our study
assays. Furthermore, it has been reported that some clinically rel-
PERFECTOR evant ANA included in the panel of these tests may be missed [7]. IIF and automated solid-phase assays have totally different test decreases with age, it is still described in 18–63% in adults [9]. was 12%, which is consistent with previous reports.
Prolonged respiratory symptoms are described both in
This study is the first to evaluate the possible association
characteristics. IIF enables the analyst to detect a broad spec-
The use of assays other than IIF is in accordance with inter-
national recommendations. However, considering that if the
between recurrence of TEF and viral bronchiolitis. As noted
children and adults following TEF repair. The etiology of pul-
trum of antibodies at one view, whereas automated immunoas-
700 689