Page 105 - Demo 1
P. 105
and stressing the bonds of that substrate in such a way as to make a parcular
reacon more likely. The key to this acvity is the shape of the enzyme.
An enzyme is specific for a parcular substrate because the enzyme
surface provides a mold that very closely fits the shape of the desired
substrate. Other molecules that do not fit as perfectly simply do not adhere to
the enzyme’s surface. The site on the enzyme surface where the substrate fits
is called the acve site. The site on the substrate that binds to an enzyme is
called the binding site. Because enzymes are not rigid, the binding of the
substrate induces the enzyme to change its shape slightly. Like all catalysts,
enzymes take part in the reacon; they do not undergo permanent changes and
so they remain unchanged at the end of the reacon.
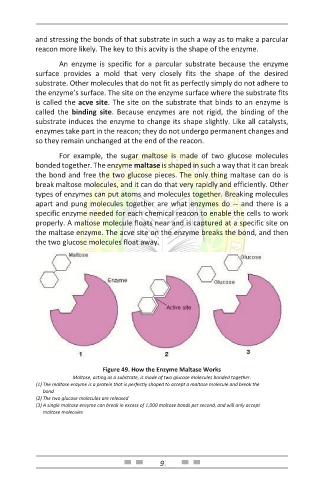
For example, the sugar maltose is made of two glucose molecules
bonded together. The enzyme maltase is shaped in such a way that it can break
the bond and free the two glucose pieces. The only thing maltase can do is
break maltose molecules, and it can do that very rapidly and efficiently. Other
types of enzymes can put atoms and molecules together. Breaking molecules
apart and pung molecules together are what enzymes do -- and there is a
specific enzyme needed for each chemical reacon to enable the cells to work
properly. A maltose molecule floats near and is captured at a specific site on
the maltase enzyme. The acve site on the enzyme breaks the bond, and then
the two glucose molecules float away.
Figure 49. How the Enzyme Maltase Works
Maltose, acting as a substrate, is made of two glucose molecules bonded together.
(1) The maltase enzyme is a protein that is perfectly shaped to accept a maltose molecule and break the
bond
(2) The two glucose molecules are released
(3) A single maltase enzyme can break in excess of 1,000 maltose bonds per second, and will only accept
maltose molecules
97