Page 32 - Covid-19 Vaccine Clinic
P. 32
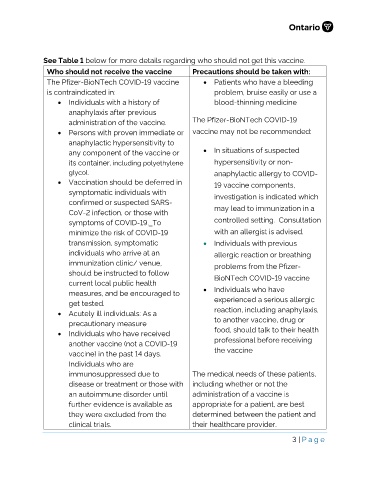
See Table 1 below for more details regarding who should not get this vaccine.
Who should not receive the vaccine Precautions should be taken with:
The Pfizer-BioNTech COVID-19 vaccine • Patients who have a bleeding
is contraindicated in: problem, bruise easily or use a
• Individuals with a history of blood-thinning medicine
anaphylaxis after previous
administration of the vaccine. The Pfizer-BioNTech COVID-19
• Persons with proven immediate or vaccine may not be recommended:
anaphylactic hypersensitivity to
any component of the vaccine or • In situations of suspected
its container, including polyethylene hypersensitivity or non-
glycol. anaphylactic allergy to COVID-
• Vaccination should be deferred in 19 vaccine components,
symptomatic individuals with investigation is indicated which
confirmed or suspected SARS-
CoV-2 infection, or those with may lead to immunization in a
symptoms of COVID-19. To controlled setting. Consultation
minimize the risk of COVID-19 with an allergist is advised.
transmission, symptomatic • Individuals with previous
individuals who arrive at an allergic reaction or breathing
immunization clinic/ venue, problems from the Pfizer-
should be instructed to follow BioNTech COVID-19 vaccine
current local public health
measures, and be encouraged to • Individuals who have
get tested. experienced a serious allergic
• Acutely ill individuals: As a reaction, including anaphylaxis,
precautionary measure to another vaccine, drug or
• Individuals who have received food, should talk to their health
another vaccine (not a COVID-19 professional before receiving
vaccine) in the past 14 days. the vaccine
Individuals who are
immunosuppressed due to The medical needs of these patients,
disease or treatment or those with including whether or not the
an autoimmune disorder until administration of a vaccine is
further evidence is available as appropriate for a patient, are best
they were excluded from the determined between the patient and
clinical trials. their healthcare provider.
3 | P ag e