Page 1004 - The Toxicology of Fishes
P. 1004
984 The Toxicology of Fishes
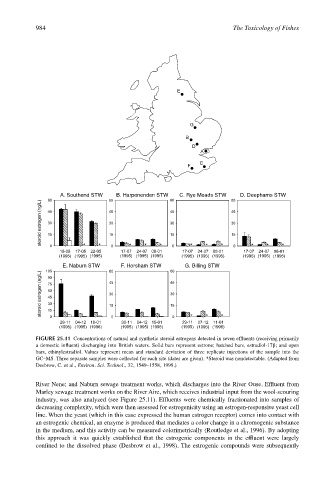
A. Southend STW B. Harpenenden STW C. Rye Meads STW D. Deephams STW
E. Naburn STW F. Horsham STW G. Billing STW
FIGURE 25.11 Concentrations of natural and synthetic steroid estrogens detected in seven effluents (receiving primarily
a domestic influent) discharging into British waters. Solid bars represent estrone; hatched bars, estradiol-17β; and open
bars, ethinylestradiol. Values represent mean and standard deviation of three replicate injections of the sample into the
GC–MS. Three separate samples were collected for each site (dates are given). *Steroid was nondetectable. (Adapted from
Desbrow, C. et al., Environ. Sci. Technol., 32, 1549–1558, 1998.)
River Nene; and Naburn sewage treatment works, which discharges into the River Ouse. Effluent from
Marley sewage treatment works on the River Aire, which receives industrial input from the wool-scouring
industry, was also analyzed (see Figure 25.11). Effluents were chemically fractionated into samples of
decreasing complexity, which were then assessed for estrogenicity using an estrogen-responsive yeast cell
line. When the yeast (which in this case expressed the human estrogen receptor) comes into contact with
an estrogenic chemical, an enzyme is produced that mediates a color change in a chromogenic substance
in the medium, and this activity can be measured colorimetrically (Routledge et al., 1996). By adopting
this approach it was quickly established that the estrogenic components in the effluent were largely
confined to the dissolved phase (Desbrow et al., 1998). The estrogenic compounds were subsequently