Page 384 - The Toxicology of Fishes
P. 384
364 The Toxicology of Fishes
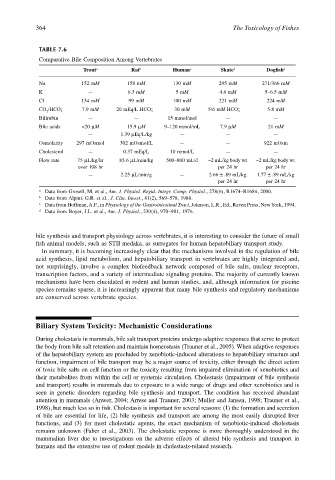
TABLE 7.6
Comparative Bile Composition Among Vertebrates
Trout a Rat b Human c Skate d Dogfish d
Na 152 mM 158 mM 130 mM 295 mM 271/366 mM
K — 6.3 mM 5 mM 4.6 mM 5–6.5 mM
Cl 134 mM 99 mM 100 mM 221 mM 224 mM
– 7.9 mM – 30 mM – 5.8 mM
CO 2 /HCO 3 20 mEq/L HCO 3 5/6 mM HCO 3
Bilirubin — — 15 mmol/mol — —
Bile acids <20 µM 15.9 µM 9–120 mmol/mL 7.9 µM 21 mM
— 1.39 µEq/L/kg — — —
Osmolarity 297 mOsmol 302 mOsmol/L — — 922 mOsm
Cholesterol — 0.37 mEq/L 10 mmol/L — —
Flow rate 75 µL/kg/hr 85.6 µL/min/kg 500–800 mL/d ~2 mL/kg body wt ~2 mL/kg body wt
over 108 hr per 24 hr per 24 hr
— 2.25 µL/min/g — 2.66 ± .89 mL/kg 1.77 ± .89 mL/kg
per 24 hr per 24 hr
a Data from Grosell, M. et al., Am. J. Physiol. Regul. Integr. Comp. Physiol., 278(6), R1674–R1684, 2000.
b Data from Alpini, G.R. et al., J. Clin. Invest., 81(2), 569–578, 1988.
c Data from Hoffman, A.F., in Physiology of the Gastrointestinal Tract, Johnson, L.R., Ed., Raven Press, New York, 1994.
d Data from Boyer, J.L. et al., Am. J. Physiol., 230(4), 970–981, 1976.
bile synthesis and transport physiology across vertebrates, it is interesting to consider the future of small
fish animal models, such as STII medaka, as surrogates for human hepatobiliary transport study.
In summary, it is becoming increasingly clear that the mechanisms involved in the regulation of bile
acid synthesis, lipid metabolism, and hepatobiliary transport in vertebrates are highly integrated and,
not surprisingly, involve a complex biofeedback network composed of bile salts, nuclear receptors,
transcription factors, and a variety of intermediate signaling proteins. The majority of currently known
mechanisms have been elucidated in rodent and human studies, and, although information for piscine
species remains sparse, it is increasingly apparent that many bile synthesis and regulatory mechanisms
are conserved across vertebrate species.
Biliary System Toxicity: Mechanistic Considerations
During cholestasis in mammals, bile salt transport proteins undergo adaptive responses that serve to protect
the body from bile salt retention and maintain homeostasis (Trauner et al., 2005). When adaptive responses
of the hepatobiliary system are precluded by xenobiotic-induced alterations to hepatobiliary structure and
function, impairment of bile transport may be a major source of toxicity, either through the direct action
of toxic bile salts on cell function or the toxicity resulting from impaired elimination of xenobiotics and
their metabolites from within the cell or systemic circulation. Cholestasis (impairment of bile synthesis
and transport) results in mammals due to exposure to a wide range of drugs and other xenobiotics and is
seen in genetic disorders regarding bile synthesis and transport. The condition has received abundant
attention in mammals (Anwer, 2004; Arrese and Trauner, 2003; Muller and Jansen, 1998; Trauner et al.,
1998), but much less so in fish. Cholestasis is important for several reasons: (1) the formation and secretion
of bile are essential for life, (2) bile synthesis and transport are among the most easily disrupted liver
functions, and (3) for most cholestatic agents, the exact mechanism of xenobiotic-induced cholestasis
remains unknown (Faber et al., 2003). The cholestatic response is more thoroughly understood in the
mammalian liver due to investigations on the adverse effects of altered bile synthesis and transport in
humans and the extensive use of rodent models in cholestasis-related research.