Page 56 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 56
Concepts in Veterinary Toxicology Chapter | 1 23
VetBooks.ir material being deposited in some regions of the respira- lifetime incidence of lung cancer whereas a population of
a two-pack-a-day cigarette smokers will experience about
tory tract while other regions are spared any exposure.
a 20% lifetime incidence of lung cancer. Consideration of
This unusual pattern of distribution of the agent is very
likely to influence the toxic responses of the airways and statistical information such as the above emphasizes the
alveoli. Thus, I am hesitant to even recommend intratra- importance of using care in interpreting the results of can-
cheal instillation for mechanistic studies, the mechanistic cer bioassays using the typical 100 animals (50 of each
information acquired may be irrelevant to the inhalation sex) per exposure level. The interpretation of the rele-
exposure situations that are of concern for people or other vance of the results of animal studies for estimating
species. human hazards will be greatly enhanced by knowledge of
It is critical that exposure-dose-response studies utilize the mechanisms involved in the toxicant causing disease
multiple exposure levels, perhaps three or four exposure in the animals.
levels. The choice of the specific exposure levels is one A key feature of the exposure response experimental
of the most important decisions to be made in planning design illustrated in Fig. 1.8 is the use of multiple sacri-
such studies. One consideration relates to the potential fice times for all exposure levels. In some cases it may be
level(s) of exposure to be encountered with intended use. possible to evaluate the functional status of organs at
Higher additional levels can be selected above this base these times, i.e., pulmonary function. In animals with
level. Selection of exposure/dose levels can also be inhalation exposure, when a respiratory tract response is
informed by the results of the kinetic studies. For exam- of concern, it may be feasible to collect bronchoalveolar
ple, it would not be desirable to use only exposure levels lavage fluid samples for analysis of biochemical and cel-
above a level at which metabolic processes are saturated. lular parameters. Most importantly, tissue samples can be
Another consideration emphasized by the EPA and NTP, collected at the multiple time periods for histopatholog-
especially when cancer is an endpoint, is to select a maxi- ical evaluation. The information obtained from the seri-
mum tolerated (MTD) dose level as the highest exposure/ ally sacrificed animals, combined with that obtained from
dose level and establish lower levels by some fraction of the terminal sacrifice animals, can provide valuable
the MTD level, perhaps 1/2 and 1/4 or 1/3 and 1/9. The insight into the progression of disease processes over the
use of an MTD has been justified on the grounds that it is course of the study. Without question, insight into the
necessary to maximize exposure to potentially observe pathogenesis of toxicant-induced disease processes will
carcinogenic responses recognizing the blunt experimental be much more complete when serial sacrifices are con-
approach (NRC, 1993). A useful review on the history of ducted than that obtained only from an evaluation of the
the use of animal bioassays to predict carcinogenicity has terminal sacrifice animals. Another option in the design of
been authored by Beyer et al. (2011). exposure response studies is to include a group of animals
The extent to which animal bioassays are a blunt at each level that are removed from further exposure at one
approach to detecting the carcinogenic potential of agents or more times postinhalation of exposure for maintenance
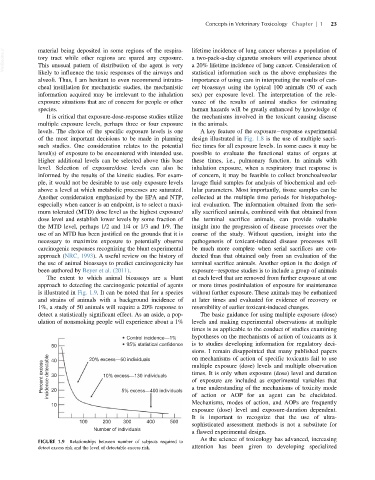
is illustrated in Fig. 1.9. It can be noted that for a species without further exposure. These animals may be euthanized
and strains of animals with a background incidence of at later times and evaluated for evidence of recovery or
1%, a study of 50 animals will require a 20% response to reversibility of earlier toxicant-induced changes.
detect a statistically significant effect. As an aside, a pop- The basic guidance for using multiple exposure (dose)
ulation of nonsmoking people will experience about a 1% levels and making experimental observations at multiple
times is as applicable to the conduct of studies examining
Control incidence—1% hypotheses on the mechanisms of action of toxicants as it
50 95% statistical confidence is to studies developing information for regulatory deci-
sions. I remain disappointed that many published papers
on mechanisms of action of specific toxicants fail to use
20% excess—50 individuals
40
Percent excess incidence detectable 30 10% excess—130 individuals multiple exposure (dose) levels and multiple observation
times. It is only when exposure (dose) level and duration
of exposure are included as experimental variables that
a true understanding of the mechanisms of toxicity mode
20
5% excess—400 individuals
of action or AOP for an agent can be elucidated.
Mechanisms, modes of action, and AOPs are frequently
10
exposure (dose) level and exposure-duration dependent.
It is important to recognize that the use of ultra-
100 200 300 400 500
sophisticated assessment methods is not a substitute for
Number of individuals
a flawed experimental design.
As the science of toxicology has advanced, increasing
FIGURE 1.9 Relationships between number of subjects required to
detect excess risk and the level of detectable excess risk. attention has been given to developing specialized