Page 1254 - Small Animal Internal Medicine, 6th Edition
P. 1254
1226 PART XI Immune-Mediated Disorders
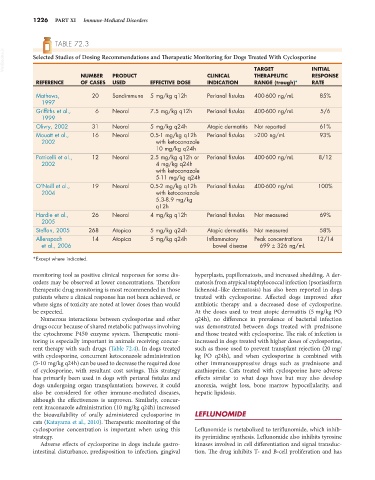
TABLE 72.3
VetBooks.ir Selected Studies of Dosing Recommendations and Therapeutic Monitoring for Dogs Treated With Cyclosporine
NUMBER PRODUCT CLINICAL TARGET INITIAL
RESPONSE
THERAPEUTIC
REFERENCE OF CASES USED EFFECTIVE DOSE INDICATION RANGE (trough)* RATE
Mathews, 20 Sandimmune 5 mg/kg q12h Perianal fistulas 400-600 ng/mL 85%
1997
Griffiths et al., 6 Neoral 7.5 mg/kg q12h Perianal fistulas 400-600 ng/mL 5/6
1999
Olivry, 2002 31 Neoral 5 mg/kg q24h Atopic dermatitis Not reported 61%
Mouatt et al., 16 Neoral 0.5-1 mg/kg q12h Perianal fistulas >200 ng/mL 93%
2002 with ketoconazole
10 mg/kg q24h
Patricelli et al., 12 Neoral 2.5 mg/kg q12h or Perianal fistulas 400-600 ng/mL 8/12
2002 4 mg/kg q24h
with ketoconazole
5-11 mg/kg q24h
O’Neill et al., 19 Neoral 0.5-2 mg/kg q12h Perianal fistulas 400-600 ng/mL 100%
2004 with ketoconazole
5.3-8.9 mg/kg
q12h
Hardie et al., 26 Neoral 4 mg/kg q12h Perianal fistulas Not measured 69%
2005
Steffan, 2005 268 Atopica 5 mg/kg q24h Atopic dermatitis Not measured 58%
Allenspach 14 Atopica 5 mg/kg q24h Inflammatory Peak concentrations 12/14
et al., 2006 bowel disease 699 ± 326 ng/mL
*Except where indicated.
monitoring tool as positive clinical responses for some dis- hyperplasia, papillomatosis, and increased shedding. A der-
orders may be observed at lower concentrations. Therefore matosis from atypical staphylococcal infection (psoriasiform
therapeutic drug monitoring is most recommended in those lichenoid–like dermatosis) has also been reported in dogs
patients where a clinical response has not been achieved, or treated with cyclosporine. Affected dogs improved after
where signs of toxicity are noted at lower doses than would antibiotic therapy and a decreased dose of cyclosporine.
be expected. At the doses used to treat atopic dermatitis (5 mg/kg PO
Numerous interactions between cyclosporine and other q24h), no difference in prevalence of bacterial infection
drugs occur because of shared metabolic pathways involving was demonstrated between dogs treated with prednisone
the cytochrome P450 enzyme system. Therapeutic moni- and those treated with cyclosporine. The risk of infection is
toring is especially important in animals receiving concur- increased in dogs treated with higher doses of cyclosporine,
rent therapy with such drugs (Table 72.4). In dogs treated such as those used to prevent transplant rejection (20 mg/
with cyclosporine, concurrent ketoconazole administration kg PO q24h), and when cyclosporine is combined with
(5-10 mg/kg q24h) can be used to decrease the required dose other immunosuppressive drugs such as prednisone and
of cyclosporine, with resultant cost savings. This strategy azathioprine. Cats treated with cyclosporine have adverse
has primarily been used in dogs with perianal fistulas and effects similar to what dogs have but may also develop
dogs undergoing organ transplantation; however, it could anorexia, weight loss, bone marrow hypocellularity, and
also be considered for other immune-mediated diseases, hepatic lipidosis.
although the effectiveness is unproven. Similarly, concur-
rent itraconazole administration (10 mg/kg q24h) increased
the bioavailability of orally administered cyclosporine in LEFLUNOMIDE
cats (Katayama et al., 2010). Therapeutic monitoring of the
cyclosporine concentration is important when using this Leflunomide is metabolized to teriflunomide, which inhib-
strategy. its pyrimidine synthesis. Leflunomide also inhibits tyrosine
Adverse effects of cyclosporine in dogs include gastro- kinases involved in cell differentiation and signal transduc-
intestinal disturbance, predisposition to infection, gingival tion. The drug inhibits T- and B-cell proliferation and has