Page 103 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 103
82 PART I The Biology and Pathogenesis of Cancer
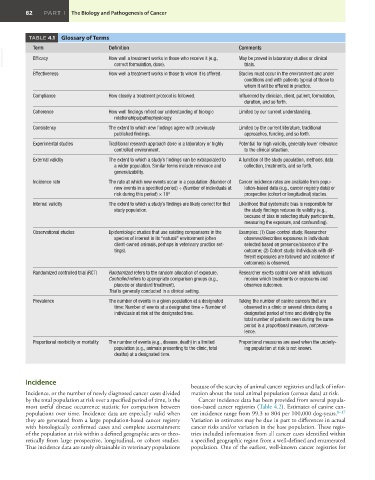
TABLE 4.1 Glossary of Terms
Term Definition Comments
VetBooks.ir Efficacy How well a treatment works in those who receive it (e.g., May be proved in laboratory studies or clinical
trials.
correct formulation, dose).
Effectiveness How well a treatment works in those to whom it is offered. Studies must occur in the environment and under
conditions and with patients typical of those to
whom it will be offered in practice.
Compliance How closely a treatment protocol is followed. Influenced by clinician, client, patient, formulation,
duration, and so forth.
Coherence How well findings reflect our understanding of biologic Limited by our current understanding.
relationships/pathophysiology.
Consistency The extent to which new findings agree with previously Limited by the current literature, traditional
published findings. approaches, funding, and so forth.
Experimental studies Traditional research approach done in a laboratory or highly Potential for high validity, generally lower relevance
controlled environment. to the clinical situation.
External validity The extent to which a study’s findings can be extrapolated to A function of the study population, methods, data
a wider population. Similar terms include relevance and collection, treatments, and so forth.
generalizability.
Incidence rate The rate at which new events occur in a population: (Number of Cancer incidence rates are available from popu-
new events in a specified period) ÷ (Number of individuals at lation-based data (e.g., cancer registry data) or
risk during this period) × 10 n prospective (cohort or longitudinal) studies.
Internal validity The extent to which a study’s findings are likely correct for that Likelihood that systematic bias is responsible for
study population. the study findings reduces its validity (e.g.,
because of bias in selecting study participants,
measuring the exposure, and confounding).
Observational studies Epidemiologic studies that use existing comparisons in the Examples: (1) Case-control study: Researcher
species of interest in its “natural” environment (often observes/describes exposures in individuals
client-owned animals, perhaps in veterinary practice set- selected based on presence/absence of the
tings). outcome; (2) Cohort study: Individuals with dif-
ferent exposures are followed and incidence of
outcome(s) is observed.
Randomized controlled trial (RCT) Randomized refers to the random allocation of exposure. Researcher exerts control over which individuals
Controlled refers to appropriate comparison groups (e.g., receive which treatments or exposures and
placebo or standard treatment). observes outcomes.
Trial is generally conducted in a clinical setting.
Prevalence The number of events in a given population at a designated Taking the number of canine cancers that are
time: Number of events at a designated time ÷ Number of observed in a clinic or several clinics during a
individuals at risk at the designated time. designated period of time and dividing by the
total number of patients seen during the same
period is a proportional measure, not preva-
lence.
Proportional morbidity or mortality The number of events (e.g., disease, death) in a limited Proportional measures are used when the underly-
population (e.g., animals presenting to the clinic, total ing population at risk is not known.
deaths) at a designated time.
Incidence
because of the scarcity of animal cancer registries and lack of infor-
Incidence, or the number of newly diagnosed cancer cases divided mation about the total animal population (census data) at risk.
by the total population at risk over a specified period of time, is the Cancer incidence data has been provided from several popula-
most useful disease occurrence statistic for comparison between tion-based cancer registries (Table 4.2). Estimates of canine can-
populations over time. Incidence data are especially valid when cer incidence range from 99.3 to 804 per 100,000 dog-years. 8–17
they are generated from a large population-based cancer registry Variation in estimates may be due in part to differences in actual
with histologically confirmed cases and complete ascertainment cancer risks and/or variation in the base population. These regis-
of the population at risk within a defined geographic area or theo- tries included information from all cancer cases identified within
retically from large prospective, longitudinal, or cohort studies. a specified geographic region from a well-defined and enumerated
True incidence data are rarely obtainable in veterinary populations population. One of the earliest, well-known cancer registries for