Page 54 - Veterinary Immunology, 10th Edition
P. 54
VetBooks.ir
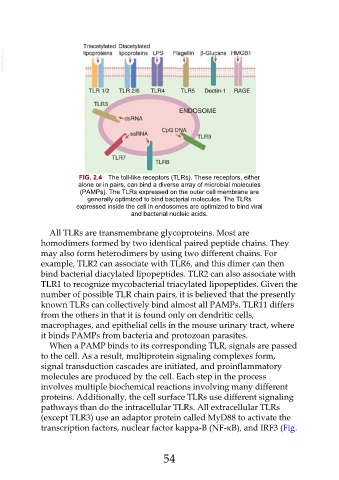
FIG. 2.4 The toll-like receptors (TLRs). These receptors, either
alone or in pairs, can bind a diverse array of microbial molecules
(PAMPs). The TLRs expressed on the outer cell membrane are
generally optimized to bind bacterial molecules. The TLRs
expressed inside the cell in endosomes are optimized to bind viral
and bacterial nucleic acids.
All TLRs are transmembrane glycoproteins. Most are
homodimers formed by two identical paired peptide chains. They
may also form heterodimers by using two different chains. For
example, TLR2 can associate with TLR6, and this dimer can then
bind bacterial diacylated lipopeptides. TLR2 can also associate with
TLR1 to recognize mycobacterial triacylated lipopeptides. Given the
number of possible TLR chain pairs, it is believed that the presently
known TLRs can collectively bind almost all PAMPs. TLR11 differs
from the others in that it is found only on dendritic cells,
macrophages, and epithelial cells in the mouse urinary tract, where
it binds PAMPs from bacteria and protozoan parasites.
When a PAMP binds to its corresponding TLR, signals are passed
to the cell. As a result, multiprotein signaling complexes form,
signal transduction cascades are initiated, and proinflammatory
molecules are produced by the cell. Each step in the process
involves multiple biochemical reactions involving many different
proteins. Additionally, the cell surface TLRs use different signaling
pathways than do the intracellular TLRs. All extracellular TLRs
(except TLR3) use an adaptor protein called MyD88 to activate the
transcription factors, nuclear factor kappa-B (NF-κB), and IRF3 (Fig.
54