Page 510 - Veterinary Immunology, 10th Edition
P. 510
VetBooks.ir
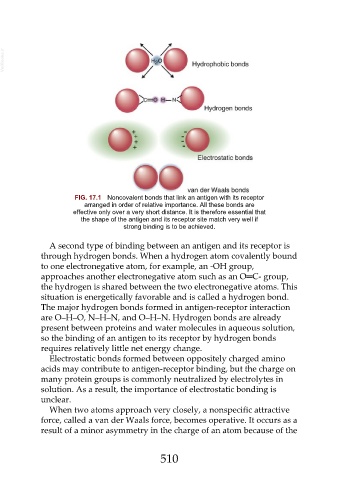
FIG. 17.1 Noncovalent bonds that link an antigen with its receptor
arranged in order of relative importance. All these bonds are
effective only over a very short distance. It is therefore essential that
the shape of the antigen and its receptor site match very well if
strong binding is to be achieved.
A second type of binding between an antigen and its receptor is
through hydrogen bonds. When a hydrogen atom covalently bound
to one electronegative atom, for example, an -OH group,
approaches another electronegative atom such as an O═C- group,
the hydrogen is shared between the two electronegative atoms. This
situation is energetically favorable and is called a hydrogen bond.
The major hydrogen bonds formed in antigen-receptor interaction
are O–H–O, N–H–N, and O–H–N. Hydrogen bonds are already
present between proteins and water molecules in aqueous solution,
so the binding of an antigen to its receptor by hydrogen bonds
requires relatively little net energy change.
Electrostatic bonds formed between oppositely charged amino
acids may contribute to antigen-receptor binding, but the charge on
many protein groups is commonly neutralized by electrolytes in
solution. As a result, the importance of electrostatic bonding is
unclear.
When two atoms approach very closely, a nonspecific attractive
force, called a van der Waals force, becomes operative. It occurs as a
result of a minor asymmetry in the charge of an atom because of the
510