Page 222 - The Toxicology of Fishes
P. 222
202 The Toxicology of Fishes
O CYP O
CH 3
N N CH 3
H
OH
SULT
CYP
HO
O
O
CH 3
N
H N CH 3
O
O
S
OH
O
SULT nonenzymatic
O
HO S O
O
O
O
N CH 3
N CH 3
H
nitrenium reactive metabolite
nontoxic sulfate conjugate
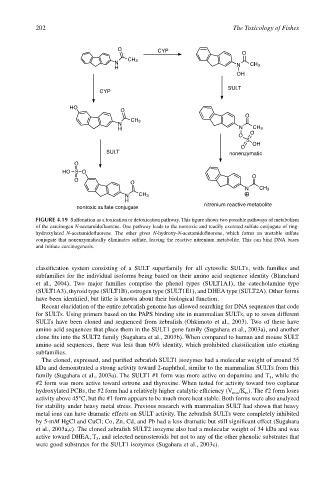
FIGURE 4.19 Sulfonation as a toxication or detoxication pathway. This figure shows two possible pathways of metabolism
of the carcinogen N-acetamidofluorene. One pathway leads to the nontoxic and readily excreted sulfate conjugate of ring-
hydroxylated N-acetamidofluorene. The other gives N-hydroxy-N-acetamidofluorene, which forms an unstable sulfate
conjugate that nonenzymatically eliminates sulfate, leaving the reactive nitrenium metabolite. This can bind DNA bases
and initiate carcinogenesis.
classification system consisting of a SULT superfamily for all cytosolic SULTs, with families and
subfamilies for the individual isoforms being based on their amino acid sequence identity (Blanchard
et al., 2004). Two major families comprise the phenol types (SULT1A1), the catecholamine type
(SULT1A3), thyroid type (SULT1B), estrogen type (SULT1E1), and DHEA type (SULT2A). Other forms
have been identified, but little is known about their biological function.
Recent elucidation of the entire zebrafish genome has allowed searching for DNA sequences that code
for SULTs. Using primers based on the PAPS binding site in mammalian SULTs, up to seven different
SULTs have been cloned and sequenced from zebrafish (Ohkimoto et al., 2003). Two of these have
amino acid sequences that place them in the SULT1 gene family (Sugahara et al., 2003a), and another
clone fits into the SULT2 family (Sugahara et al., 2003b). When compared to human and mouse SULT
amino acid sequences, there was less than 60% identity, which prohibited classification into existing
subfamilies.
The cloned, expressed, and purified zebrafish SULT1 isozymes had a molecular weight of around 35
kDa and demonstrated a strong activity toward 2-naphthol, similar to the mammalian SULTs from this
family (Sugahara et al., 2003a). The SULT1 #1 form was more active on dopamine and T , while the
3
#2 form was more active toward estrone and thyroxine. When tested for activity toward two coplanar
hydroxylated PCBs, the #2 form had a relatively higher catalytic efficiency (V max /K ). The #2 form loses
m
activity above 45°C, but the #1 form appears to be much more heat stable. Both forms were also analyzed
for stability under heavy metal stress. Previous research with mammalian SULT had shown that heavy
metal ions can have dramatic effects on SULT activity. The zebrafish SULTs were completely inhibited
by 5-mM HgCl and CuCl; Co, Zn, Cd, and Pb had a less dramatic but still significant effect (Sugahara
et al., 2003a,c). The cloned zebrafish SULT2 isozyme also had a molecular weight of 34 kDa and was
active toward DHEA, T , and selected neurosteroids but not to any of the other phenolic substrates that
3
were good substrates for the SULT1 isozymes (Sugahara et al., 2003c).