Page 343 - ABCTE Study Guide_Neat
P. 343
and the metal atoms are bound by the electrical attraction between the metal cations and the electrons.
Metals conduct electricity well because the electrons are able to move easily from one atom to another.
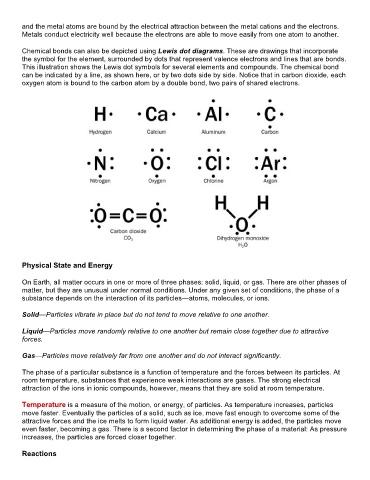
Chemical bonds can also be depicted using Lewis dot diagrams. These are drawings that incorporate
the symbol for the element, surrounded by dots that represent valence electrons and lines that are bonds.
This illustration shows the Lewis dot symbols for several elements and compounds. The chemical bond
can be indicated by a line, as shown here, or by two dots side by side. Notice that in carbon dioxide, each
oxygen atom is bound to the carbon atom by a double bond, two pairs of shared electrons.
Physical State and Energy
On Earth, all matter occurs in one or more of three phases: solid, liquid, or gas. There are other phases of
matter, but they are unusual under normal conditions. Under any given set of conditions, the phase of a
substance depends on the interaction of its particles—atoms, molecules, or ions.
Solid—Particles vibrate in place but do not tend to move relative to one another.
Liquid—Particles move randomly relative to one another but remain close together due to attractive
forces.
Gas—Particles move relatively far from one another and do not interact significantly.
The phase of a particular substance is a function of temperature and the forces between its particles. At
room temperature, substances that experience weak interactions are gases. The strong electrical
attraction of the ions in ionic compounds, however, means that they are solid at room temperature.
Temperature is a measure of the motion, or energy, of particles. As temperature increases, particles
move faster. Eventually the particles of a solid, such as ice, move fast enough to overcome some of the
attractive forces and the ice melts to form liquid water. As additional energy is added, the particles move
even faster, becoming a gas. There is a second factor in determining the phase of a material: As pressure
increases, the particles are forced closer together.
Reactions