Page 96 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 96
82 SECTION I Basic Principles
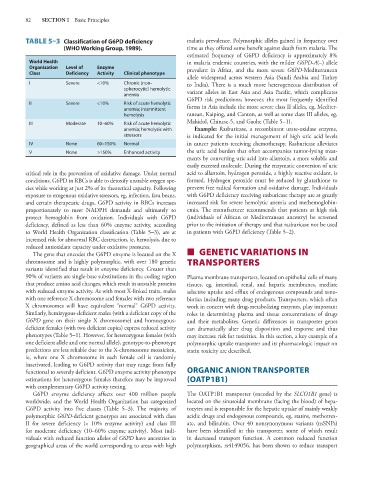
TABLE 5–3 Classification of G6PD deficiency malaria prevalence. Polymorphic alleles gained in frequency over
(WHO Working Group, 1989). time as they offered some benefit against death from malaria. The
estimated frequency of G6PD deficiency is approximately 8%
World Health in malaria endemic countries, with the milder G6PD-A(–) allele
Organization Level of Enzyme prevalent in Africa, and the more severe G6PD-Mediterranean
Class Deficiency Activity Clinical phenotype
allele widespread across western Asia (Saudi Arabia and Turkey
I Severe <10% Chronic (non- to India). There is a much more heterogeneous distribution of
spherocytic) hemolytic variant alleles in East Asia and Asia Pacific, which complicates
anemia
G6PD risk predictions; however, the most frequently identified
II Severe <10% Risk of acute hemolytic forms in Asia include the more severe class II alleles, eg, Mediter-
anemia; intermittent
hemolysis ranean, Kaiping, and Canton, as well as some class III alleles, eg,
III Moderate 10–60% Risk of acute hemolytic Mahidol, Chinese-5, and Gaohe (Table 5–1).
anemia; hemolysis with Example: Rasburicase, a recombinant urate-oxidase enzyme,
stressors is indicated for the initial management of high uric acid levels
IV None 60–150% Normal in cancer patients receiving chemotherapy. Rasburicase alleviates
V None >150% Enhanced activity the uric acid burden that often accompanies tumor-lysing treat-
ments by converting uric acid into allantoin, a more soluble and
easily excreted molecule. During the enzymatic conversion of uric
critical role in the prevention of oxidative damage. Under normal acid to allantoin, hydrogen peroxide, a highly reactive oxidant, is
conditions, G6PD in RBCs is able to detoxify unstable oxygen spe- formed. Hydrogen peroxide must be reduced by glutathione to
cies while working at just 2% of its theoretical capacity. Following prevent free radical formation and oxidative damage. Individuals
exposure to exogenous oxidative stressors, eg, infection, fava beans, with G6PD deficiency receiving rasburicase therapy are at greatly
and certain therapeutic drugs, G6PD activity in RBCs increases increased risk for severe hemolytic anemia and methemoglobin-
proportionately to meet NADPH demands and ultimately to emia. The manufacturer recommends that patients at high risk
protect hemoglobin from oxidation. Individuals with G6PD (individuals of African or Mediterranean ancestry) be screened
deficiency, defined as less than 60% enzyme activity, according prior to the initiation of therapy and that rasburicase not be used
to World Health Organization classification (Table 5–3), are at in patients with G6PD deficiency (Table 5–2).
increased risk for abnormal RBC destruction, ie, hemolysis, due to
reduced antioxidant capacity under oxidative pressures.
The gene that encodes the G6PD enzyme is located on the X ■ GENETIC VARIATIONS IN
chromosome and is highly polymorphic, with over 180 genetic TRANSPORTERS
variants identified that result in enzyme deficiency. Greater than
90% of variants are single-base substitutions in the coding region Plasma membrane transporters, located on epithelial cells of many
that produce amino acid changes, which result in unstable proteins tissues, eg, intestinal, renal, and hepatic membranes, mediate
with reduced enzyme activity. As with most X-linked traits, males selective uptake and efflux of endogenous compounds and xeno-
with one reference X chromosome and females with two reference biotics including many drug products. Transporters, which often
X chromosomes will have equivalent “normal” G6PD activity. work in concert with drug-metabolizing enzymes, play important
Similarly, hemizygous-deficient males (with a deficient copy of the roles in determining plasma and tissue concentrations of drugs
G6PD gene on their single X chromosome) and homozygous- and their metabolites. Genetic differences in transporter genes
deficient females (with two deficient copies) express reduced activity can dramatically alter drug disposition and response and thus
phenotypes (Table 5–1). However, for heterozygous females (with may increase risk for toxicities. In this section, a key example of a
one deficient allele and one normal allele), genotype-to-phenotype polymorphic uptake transporter and its pharmacologic impact on
predictions are less reliable due to the X-chromosome mosaicism, statin toxicity are described.
ie, where one X chromosome in each female cell is randomly
inactivated, leading to G6PD activity that may range from fully
functional to severely deficient. G6PD enzyme activity phenotype ORGANIC ANION TRANSPORTER
estimations for heterozygous females therefore may be improved (OATP1B1)
with complementary G6PD activity testing.
G6PD enzyme deficiency affects over 400 million people The OATP1B1 transporter (encoded by the SLCO1B1 gene) is
worldwide, and the World Health Organization has categorized located on the sinusoidal membrane (facing the blood) of hepa-
G6PD activity into five classes (Table 5–3). The majority of tocytes and is responsible for the hepatic uptake of mainly weakly
polymorphic G6PD-deficient genotypes are associated with class acidic drugs and endogenous compounds, eg, statins, methotrex-
II for severe deficiency (< 10% enzyme activity) and class III ate, and bilirubin. Over 40 nonsynonymous variants (nsSNPs)
for moderate deficiency (10–60% enzyme activity). Most indi- have been identified in this transporter, some of which result
viduals with reduced function alleles of G6PD have ancestries in in decreased transport function. A common reduced function
geographical areas of the world corresponding to areas with high polymorphism, rs4149056, has been shown to reduce transport