Page 156 - Ilmu Tanah
P. 156
The Chemistry and Fertility of Soils under Tropical Weeds 143
pH with high concentrations of heavy metals, particularly if the concentrations of
the precipitating agents like carbonate and sulphate ions in soil water are high
(Singh and Sekhon, 1977; Brummer et al., 1983).
Some soil properties may affect the magnitude of the respective forms of
heavy metals in the soil environment. Soil pH is repeatedly reported to be the
most important factor (Salam and Helmke, 1998; Salam, 2017; Salam, 2019). As
discussed previously the increase in soil pH may lower the concentrations of free
ions and total dissolved heavy metals in soil solution (Fig. 8.2). At high
concentration of heavy metals, parts of the heavy metals are precipitated forming
-
secondary minerals due to the increase in the concentration of OH as shown in Fig.
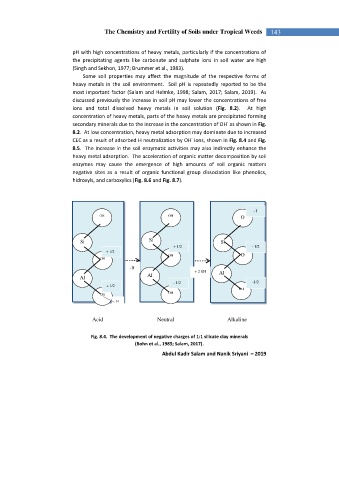
8.2. At low concentration, heavy metal adsorption may dominate due to increased
-
CEC as a result of adsorbed H neutralization by OH ions, shown in Fig. 8.4 and Fig.
8.5. The increase in the soil enzymatic activities may also indirectly enhance the
heavy metal adsorption. The acceleration of organic matter decomposition by soil
enzymes may cause the emergence of high amounts of soil organic matters
negative sites as a result of organic functional group dissociation like phenolics,
hidroxyls, and carboxylics (Fig. 8.6 and Fig. 8.7).
-1
OH OH O
Si Si + 1/2 Si - 1/2
+ 1/2 OH O
OH
-H
Al Al + 2 0H Al
- 1/2 -1/2
+ 1/2 OH
OH OH
H
Acid Neutral Alkaline
Fig. 8.4. The development of negative charges of 1:1 silicate clay minerals
(Bohn et al., 1985; Salam, 2017).
Abdul Kadir Salam and Nanik Sriyani – 2019