Page 23 - Ilmu Tanah Book
P. 23
10 The Chemistry and Fertility of Soils under Tropical Weeds
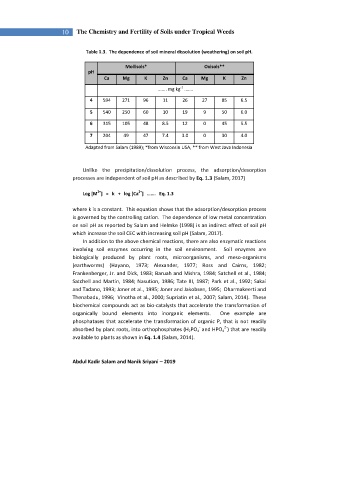
Table 1.3. The dependence of soil mineral dissolution (weathering) on soil pH.
Mollisols* Oxisols**
pH
Ca Mg K Zn Ca Mg K Zn
-1
....... mg kg .......
4 594 271 96 11 26 27 85 6.5
5 540 250 60 10 19 9 50 6.0
6 315 105 48 8.5 12 0 45 5.5
7 204 49 47 7.4 3.0 0 30 4.0
Adapted from Salam (1989); *from Wisconsin USA, ** from West Java Indonesia
Unlike the precipitation/dissolution process, the adsorption/desorption
processes are independent of soil pH as described by Eq. 1.3 (Salam, 2017)
2+ 2+
Log [M ] = k + log [Ca ] ……. Eq. 1.3
where k is a constant. This equation shows that the adsorption/desorption process
is governed by the controlling cation. The dependence of low metal concentration
on soil pH as reported by Salam and Helmke (1998) is an indirect effect of soil pH
which increase the soil CEC with increasing soil pH (Salam, 2017).
In addition to the above chemical reactions, there are also enzymatic reactions
involving soil enzymes occurring in the soil environment. Soil enzymes are
biologically produced by plant roots, microorganisms, and meso-organisms
(earthworms) (Hayano, 1973; Alexander, 1977; Ross and Cairns, 1982;
Frankenberger, Jr. and Dick, 1983; Baruah and Mishra, 1984; Satchell et al., 1984;
Satchell and Martin, 1984; Nasution, 1986; Tate III, 1987; Park et al., 1992; Sakai
and Tadano, 1993; Joner et al., 1995; Joner and Jakobsen, 1995; Dharmakeerti and
Thenabadu, 1996; Vinotha et al., 2000; Supriatin et al., 2007; Salam, 2014). These
biochemical compounds act as bio-catalysts that accelerate the transformation of
organically bound elements into inorganic elements. One example are
phosphatases that accelerate the transformation of organic P, that is not readily
-
2-
absorbed by plant roots, into orthophosphates (H 2 PO 4 and HPO 4 ) that are readily
available to plants as shown in Eq. 1.4 (Salam, 2014).
Abdul Kadir Salam and Nanik Sriyani – 2019