Page 65 - e-modul laju reaksi fix virtual lab
P. 65
E-MODUL BERBASIS DISCOVERY LEARNING
LAJU REAKSI
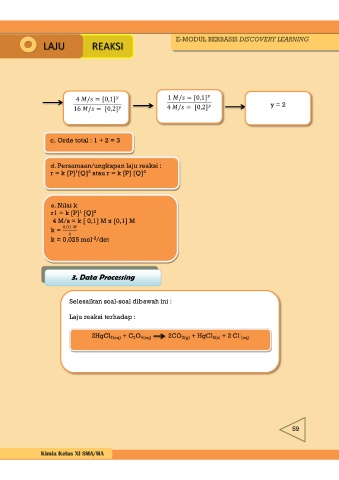
4 / = [0,1] 1 / = [0,1] y = 2
16 / = [0,2] 4 / = [0,2]
c. Orde total : 1 + 2 = 3
d. Persamaan/ungkapan laju reaksi :
r = k [P] [Q] atau r = k [P] [Q] 2
2
1
e. Nilai k 1 2
r1 = k [P] [Q]
4 M/s = k [ 0,1] M x [0,1] M
k = 0,01
4 -2
k = 0,025 mol /det
3. Data Processing
Selesaikan soal-soal dibawah ini :
Laju reaksi terhadap :
59
Kimia Kelas XI SMA/MA