Page 2 - trial
P. 2
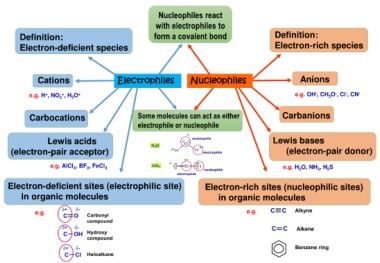
Nucleophiles react
with electrophiles to
Definition: form a covalent bond Definition:
Electron-deficient species Electron-rich species
Cations Electrophiles Nucleophiles Anions
-
-
-
+
+
e.g. H , NO , H O + e.g. OH , CH O , Cl , CN -
3
3
2
Carbocations Some molecules can act as either Carbanions
electrophile or nucleophile
Lewis acids Lewis bases
(electron-pair acceptor) (electron-pair donor)
e.g. AlCl , BF , FeCl 3 e.g. H O, NH , H S
3
3
2
3
2
Electron-deficient sites (electrophilic site) Electron-rich sites (nucleophilic sites)
in organic molecules in organic molecules
e.g. e.g.