Page 141 - ro membanes
P. 141
124 6. CONDITIONING OF SALINE WATER
It is important to point out that the results of this type of software are very sensitive to the accuracy and completeness of the source water quality analysis. Sometimes parameters such as barium, strontium, and fluoride are not measured in the source water and assumed concentrations are used instead. Such practices may often cause the projection results to be inaccurate. General steps to determine the limiting salts and to manually calculate allowable permeate recovery for saline waters of given mineral content are illustrated elsewhere (AWWA, 2007).
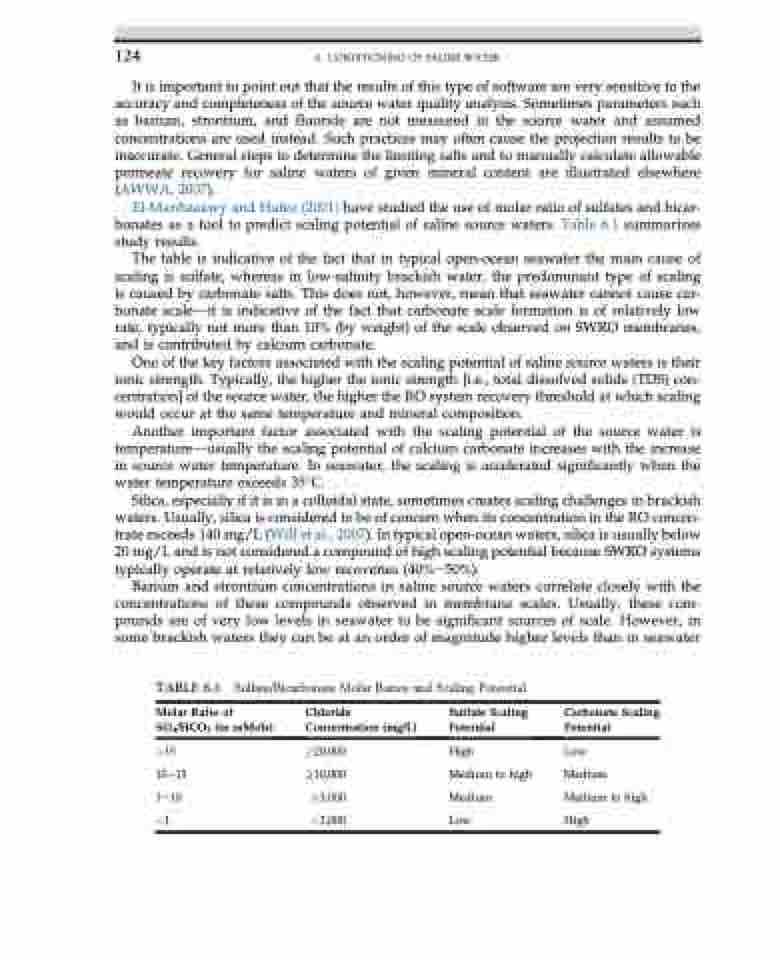
El-Manhaeawy and Hafez (2001) have studied the use of molar ratio of sulfates and bicar- bonates as a tool to predict scaling potential of saline source waters. Table 6.1 summarizes study results.
The table is indicative of the fact that in typical open-ocean seawater the main cause of scaling is sulfate, whereas in low-salinity brackish water, the predominant type of scaling is caused by carbonate salts. This does not, however, mean that seawater cannot cause car- bonate scaledit is indicative of the fact that carbonate scale formation is of relatively low rate, typically not more than 10% (by weight) of the scale observed on SWRO membranes, and is contributed by calcium carbonate.
One of the key factors associated with the scaling potential of saline source waters is their ionic strength. Typically, the higher the ionic strength [i.e., total dissolved solids (TDS) con- centration] of the source water, the higher the RO system recovery threshold at which scaling would occur at the same temperature and mineral composition.
Another important factor associated with the scaling potential of the source water is temperaturedusually the scaling potential of calcium carbonate increases with the increase in source water temperature. In seawater, the scaling is accelerated significantly when the water temperature exceeds 35C.
Silica, especially if it is in a colloidal state, sometimes creates scaling challenges in brackish waters. Usually, silica is considered to be of concern when its concentration in the RO concen- trate exceeds 140 mg/L (Wilf et al., 2007). In typical open-ocean waters, silica is usually below 20 mg/L and is not considered a compound of high scaling potential because SWRO systems typically operate at relatively low recoveries (40%e50%).
Barium and strontium concentrations in saline source waters correlate closely with the concentrations of these compounds observed in membrane scales. Usually, these com- pounds are of very low levels in seawater to be significant sources of scale. However, in some brackish waters they can be at an order of magnitude higher levels than in seawater
TABLE 6.1 Sulfate/Bicarbonate Molar Ratios and Scaling Potential
Molar Ratio of SO4/HCO3 (in mMols)
>15 10e15 1e10 <1
Chloride Concentration (mg/L)
!20,000 !10,000 !3,000 <3,000
Sulfate Scaling Potential
High
Medium to high Medium
Low
Carbonate Scaling Potential
Low
Medium Medium to high High