Page 47 - 2019秋季手冊內頁-ebook測試
P. 47
18
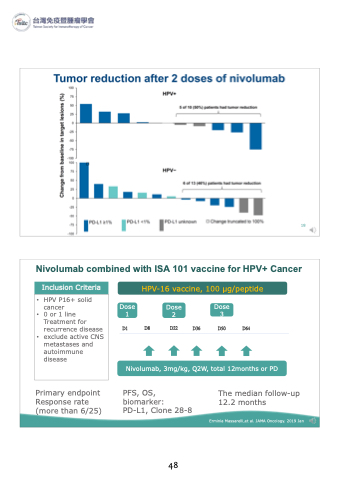
Nivolumab combined with ISA 101 vaccine for HPV+ Cancer
Inclusion Criteria
• HPV P16+ solid cancer
• 0 or 1 line Treatment for recurrence disease
• exclude active CNS metastases and autoimmune disease
HPV-16 vaccine, 100 μg/peptide
Dose Dose Dose 123
D1 D8 D22 D36 D50 D64
Nivolumab, 3mg/kg, Q2W, total 12months or PD
Primary endpoint Response rate (more than 6/25)
PFS, OS, biomarker:
PD-L1, Clone 28-8
The median follow-up 12.2 months
Erminia Massarelli,et al. JAMA Oncology. 2019 Jan
48