Page 370 - SUBSEC October 2017_Neat
P. 370
- 11 -
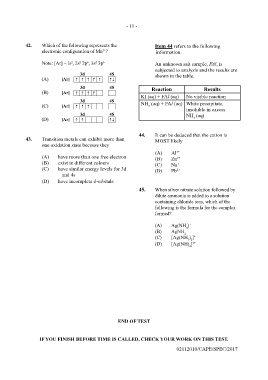
42. Which of the following represents the Item 44 refers to the following
3+
electronic configuration of Mn ? information.
Note: [Ar] = ls , 2s 2p , 3s 3p 6 An unknown salt sample, FAl, is
6
2
2
2
subjected to analysis and the results are
shown in the table.
Reaction Results
KI (aq) + FAl (aq) No visible reaction
NH (aq) + FAl (aq) White precipitate,
3
insoluble in excess
NH (aq)
3
44. It can be deduced that the cation is
43. Transition metals can exhibit more than MOST likely
one oxidation state because they
(A) Al 3+
(A) have more than one free electron (B) Zn 2+
(B) exist in different colours (C) Na +
(C) have similar energy levels for 3d (D) Pb 2+
and 4s
(D) have incomplete d-orbitals
45. When silver nitrate solution followed by
dilute ammonia is added to a solution
containing chloride ions, which of the
following is the formula for the complex
formed?
(A) Ag(NH ) +
4
(B) AgNH 2
(C) [Ag(NH ) ] +
3 2
(D) [Ag(NH) ] 2+
4
END OF TEST
IF YOU FINISH BEFORE TIME IS CALLED, CHECK YOUR WORK ON THIS TEST.
02112010/CAPE/SPEC/2017