Page 57 - HSP COVID Rapid Testing Booklet RV6
P. 57
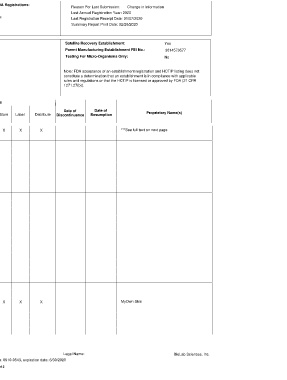
DEPARTMENT OF HEALTH AND HUMAN SERVICES Other FDA Registrations:
Reason For Last Submission: Change in Information
PUBLIC HEALTH SERVICE Blood:
FOOD AND DRUG ADMINISTRATION FEI: 3014573577 Devices: Last Annual Registration Year: 2020
ESTABLISHMENT REGISTRATION AND LISTING FOR HUMAN CELLS, Last Registration Receipt Date: 01/07/2020
Drugs: Summary Report Print Date: 02/26/2020
TISSUES, AND CELLULAR AND TISSUE-BASED PRODUCTS
DESCRIBED IN 21 CFR 1271.10
Legal Name and Location: Reporting Official: Satellite Recovery Establishment: Yes
Parent Manufacturing Establishment FEI No.: 3014573577
BioLab Sciences, Inc. Robert D Maguire, President & CEO
13825 N Northsight Boulevard Testing For Micro-Organisms Only:
7662 E Gray Rd No
Suite 107 Suite 101
Scottsdale, Arizona 85260
Note: FDA acceptance of an establishment registration and HCT/P listing does not
USA constitute a determination that an establishment is in compliance with applicable
Scottsdale, Arizona 85260 Phone: 480-688-5760 Ext. rules and regulations or that the HCT/P is licensed or approved by FDA (21 CFR
USA 1271.27(b)).
bmaguire@biolabsciences.net
Phone: 480-688-5760 Ext.:
Establishment Functions
Date of Date of
HCT/P(s) Donor Type(s) Recover Screen Donor Testing Package Process Store Label Distribute Discontinuance Resumption Proprietary Name(s)
Amniotic Membrane X X X X X ***See full text on next page.
Blood Vessel
Bone
Cardiac Tissue - non-valved
Cartilage
Cornea
Dura Mater
Embryo
Fascia
Heart Valve
HPC Apheresis
HPC Cord Blood
Ligament
Nerve Tissue
Oocyte
Ovarian Tissue
Pancreatic Islet Cells - autologous
Parathyroid
Pericardium
Peripheral Blood Mononuclear Cells
Peritoneal Membrane
Sclera
Semen
Skin X X X X X MyOwn Skin
Tendon
Testicular Tissue
Tooth Pulp
Umbilical Cord Tissue
FEI: 3014573577 Legal Name: BioLab Sciences, Inc.
FDA information collection OMB Control number: 0910-0543, expiration date: 6/30/2020
Page 1 of 2