Page 20 - Demo
P. 20
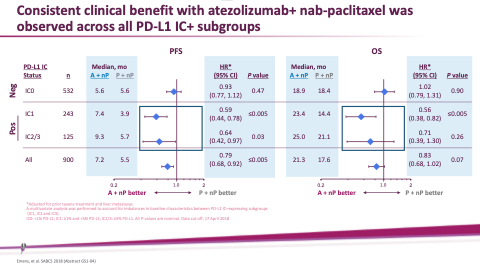
Consistent clinical benefit with atezolizumab+ nab-paclitaxel was observed across all PD-L1 IC+ subgroups
PFS
OS
Median, mo
A + nP P + nP 5.6 5.6
7.4 3.9 9.3 5.7
7.2 5.5
HR*
(95% CI) P value
0.93 (0.77, 1.12)
0.59 (0.44, 0.78)
0.64 (0.42, 0.97)
≤0.005 0.03
0.79 (0.68, 0.92)
≤0.005
0.47
Median, mo
A + nP P + nP 18.9 18.4
23.4 14.4 25.0 21.1
21.3 17.6
HR*
(95% CI) P value
1.02 (0.79, 1.31)
0.56 (0.38, 0.82)
0.71 (0.39, 1.30)
≤0.005 0.26
0.83 (0.68, 1.02)
0.07
0.90
PD-L1 IC
Status n
IC0 532
IC1 243
IC2/3 125 All 900
0.2 1.0 A + nP better
2
P + nP better
0.2
A + nP better
1.0 2
P + nP better
*Adjusted for prior taxane treatment and liver metastases
A multivariate analysis was performed to account for imbalances in baseline characteristics between PD-L1 IC–expressing subgroups
(IC1, IC2 and IC3)
IC0: <1% PD-L1; IC1: ≥1% and <5% PD-L1; IC2/3: ≥5% PD-L1. All P-values are nominal. Data cut off: 17 April 2018
Emens, et al. SABCS 2018 (Abstract GS1-04)
Pos Neg