Page 111 - pfizervax
P. 111
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
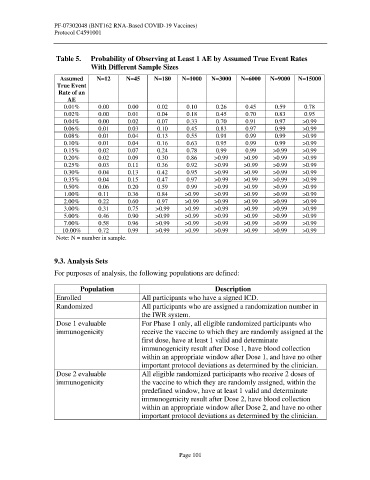
Table 5. Probability of Observing at Least 1 AE by Assumed True Event Rates
With Different Sample Sizes
Assumed N=12 N=45 N=180 N=1000 N=3000 N=6000 N=9000 N=15000
True Event
Rate of an
AE
0.01% 0.00 0.00 0.02 0.10 0.26 0.45 0.59 0.78
0.02% 0.00 0.01 0.04 0.18 0.45 0.70 0.83 0.95
0.04% 0.00 0.02 0.07 0.33 0.70 0.91 0.97 >0.99
0.06% 0.01 0.03 0.10 0.45 0.83 0.97 0.99 >0.99
0.08% 0.01 0.04 0.13 0.55 0.91 0.99 0.99 >0.99
0.10% 0.01 0.04 0.16 0.63 0.95 0.99 0.99 >0.99
0.15% 0.02 0.07 0.24 0.78 0.99 0.99 >0.99 >0.99
0.20% 0.02 0.09 0.30 0.86 >0.99 >0.99 >0.99 >0.99
0.25% 0.03 0.11 0.36 0.92 >0.99 >0.99 >0.99 >0.99
0.30% 0.04 0.13 0.42 0.95 >0.99 >0.99 >0.99 >0.99
0.35% 0.04 0.15 0.47 0.97 >0.99 >0.99 >0.99 >0.99
0.50% 0.06 0.20 0.59 0.99 >0.99 >0.99 >0.99 >0.99
1.00% 0.11 0.36 0.84 >0.99 >0.99 >0.99 >0.99 >0.99
2.00% 0.22 0.60 0.97 >0.99 >0.99 >0.99 >0.99 >0.99
3.00% 0.31 0.75 >0.99 >0.99 >0.99 >0.99 >0.99 >0.99
5.00% 0.46 0.90 >0.99 >0.99 >0.99 >0.99 >0.99 >0.99
7.00% 0.58 0.96 >0.99 >0.99 >0.99 >0.99 >0.99 >0.99
10.00% 0.72 0.99 >0.99 >0.99 >0.99 >0.99 >0.99 >0.99
Note: N = number in sample.
9.3. Analysis Sets
For purposes of analysis, the following populations are defined:
Population Description
Enrolled All participants who have a signed ICD.
Randomized All participants who are assigned a randomization number in
the IWR system.
Dose 1 evaluable For Phase 1 only, all eligible randomized participants who
immunogenicity receive the vaccine to which they are randomly assigned at the
first dose, have at least 1 valid and determinate
immunogenicity result after Dose 1, have blood collection
within an appropriate window after Dose 1, and have no other
important protocol deviations as determined by the clinician.
Dose 2 evaluable All eligible randomized participants who receive 2 doses of
immunogenicity the vaccine to which they are randomly assigned, within the
predefined window, have at least 1 valid and determinate
immunogenicity result after Dose 2, have blood collection
within an appropriate window after Dose 2, and have no other
important protocol deviations as determined by the clinician.
Page 101