Page 133 - pfizervax
P. 133
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
10.2. Appendix 2: Clinical Laboratory Tests
The following safety laboratory tests will be performed at times defined in the SoA section of
this protocol. Additional laboratory results may be reported on these samples as a result of
the method of analysis or the type of analyzer used by the clinical laboratory, or as derived
from calculated values. These additional tests would not require additional collection of
blood. Unscheduled clinical laboratory measurements may be obtained at any time during
the study to assess any perceived safety issues.
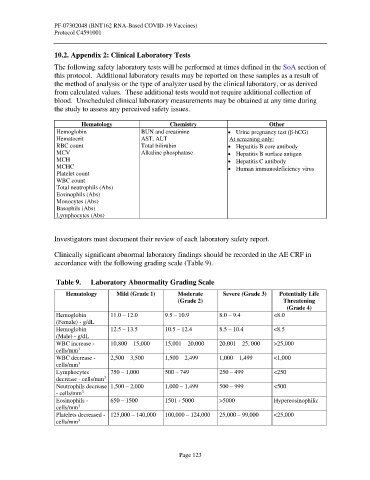
Hematology Chemistry Other
Hemoglobin BUN and creatinine • Urine pregnancy test (β-hCG)
Hematocrit AST, ALT At screening only:
RBC count Total bilirubin • Hepatitis B core antibody
MCV Alkaline phosphatase • Hepatitis B surface antigen
MCH • Hepatitis C antibody
MCHC • Human immunodeficiency virus
Platelet count
WBC count
Total neutrophils (Abs)
Eosinophils (Abs)
Monocytes (Abs)
Basophils (Abs)
Lymphocytes (Abs)
Investigators must document their review of each laboratory safety report.
Clinically significant abnormal laboratory findings should be recorded in the AE CRF in
accordance with the following grading scale (Table 9).
Table 9. Laboratory Abnormality Grading Scale
Hematology Mild (Grade 1) Moderate Severe (Grade 3) Potentially Life
(Grade 2) Threatening
(Grade 4)
Hemoglobin 11.0 – 12.0 9.5 – 10.9 8.0 – 9.4 <8.0
(Female) - g/dL
Hemoglobin 12.5 – 13.5 10.5 – 12.4 8.5 – 10.4 <8.5
(Male) - g/dL
WBC increase - 10,800 – 15,000 15,001 – 20,000 20,001 – 25, 000 >25,000
3
cells/mm
WBC decrease - 2,500 – 3,500 1,500 – 2,499 1,000 – 1,499 <1,000
3
cells/mm
Lymphocytes 750 – 1,000 500 – 749 250 – 499 <250
3
decrease - cells/mm
Neutrophils decrease 1,500 – 2,000 1,000 – 1,499 500 – 999 <500
3
- cells/mm
Eosinophils - 650 – 1500 1501 - 5000 >5000 Hypereosinophilic
3
cells/mm
Platelets decreased - 125,000 – 140,000 100,000 – 124,000 25,000 – 99,000 <25,000
3
cells/mm
Page 123