Page 44 - pfizervax
P. 44
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
a
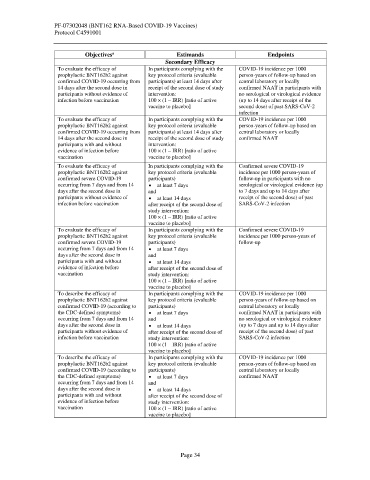
Objectives Estimands Endpoints
Secondary Efficacy
To evaluate the efficacy of In participants complying with the COVID-19 incidence per 1000
prophylactic BNT162b2 against key protocol criteria (evaluable person-years of follow-up based on
confirmed COVID-19 occurring from participants) at least 14 days after central laboratory or locally
14 days after the second dose in receipt of the second dose of study confirmed NAAT in participants with
participants without evidence of intervention: no serological or virological evidence
infection before vaccination 100 × (1 – IRR) [ratio of active (up to 14 days after receipt of the
vaccine to placebo] second dose) of past SARS-CoV-2
infection
To evaluate the efficacy of In participants complying with the COVID-19 incidence per 1000
prophylactic BNT162b2 against key protocol criteria (evaluable person-years of follow-up based on
confirmed COVID-19 occurring from participants) at least 14 days after central laboratory or locally
14 days after the second dose in receipt of the second dose of study confirmed NAAT
participants with and without intervention:
evidence of infection before 100 × (1 – IRR) [ratio of active
vaccination vaccine to placebo]
To evaluate the efficacy of In participants complying with the Confirmed severe COVID-19
prophylactic BNT162b2 against key protocol criteria (evaluable incidence per 1000 person-years of
confirmed severe COVID-19 participants) follow-up in participants with no
occurring from 7 days and from 14 • at least 7 days serological or virological evidence (up
days after the second dose in and to 7 days and up to 14 days after
participants without evidence of • at least 14 days receipt of the second dose) of past
infection before vaccination after receipt of the second dose of SARS-CoV-2 infection
study intervention:
100 × (1 – IRR) [ratio of active
vaccine to placebo]
To evaluate the efficacy of In participants complying with the Confirmed severe COVID-19
prophylactic BNT162b2 against key protocol criteria (evaluable incidence per 1000 person-years of
confirmed severe COVID-19 participants) follow-up
occurring from 7 days and from 14 • at least 7 days
days after the second dose in and
participants with and without • at least 14 days
evidence of infection before after receipt of the second dose of
vaccination study intervention:
100 × (1 – IRR) [ratio of active
vaccine to placebo]
To describe the efficacy of In participants complying with the COVID-19 incidence per 1000
prophylactic BNT162b2 against key protocol criteria (evaluable person-years of follow-up based on
confirmed COVID-19 (according to participants) central laboratory or locally
the CDC-defined symptoms) • at least 7 days confirmed NAAT in participants with
occurring from 7 days and from 14 and no serological or virological evidence
days after the second dose in • at least 14 days (up to 7 days and up to 14 days after
participants without evidence of after receipt of the second dose of receipt of the second dose) of past
infection before vaccination study intervention: SARS-CoV-2 infection
100 × (1 – IRR) [ratio of active
vaccine to placebo]
To describe the efficacy of In participants complying with the COVID-19 incidence per 1000
prophylactic BNT162b2 against key protocol criteria (evaluable person-years of follow-up based on
confirmed COVID-19 (according to participants) central laboratory or locally
the CDC-defined symptoms) • at least 7 days confirmed NAAT
occurring from 7 days and from 14 and
days after the second dose in • at least 14 days
participants with and without after receipt of the second dose of
evidence of infection before study intervention:
vaccination 100 × (1 – IRR) [ratio of active
vaccine to placebo]
Page 34