Page 56 - pfizervax
P. 56
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
6.1. Study Intervention(s) Administered
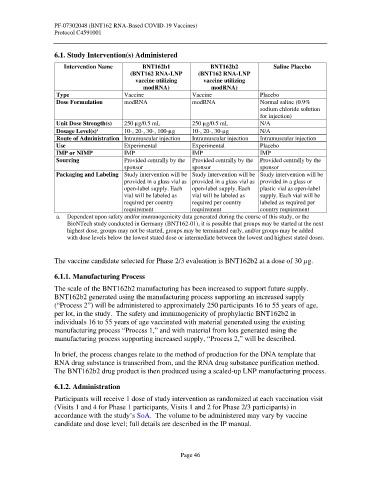
Intervention Name BNT162b1 BNT162b2 Saline Placebo
(BNT162 RNA-LNP (BNT162 RNA-LNP
vaccine utilizing vaccine utilizing
modRNA) modRNA)
Type Vaccine Vaccine Placebo
Dose Formulation modRNA modRNA Normal saline (0.9%
sodium chloride solution
for injection)
Unit Dose Strength(s) 250 µg/0.5 mL 250 µg/0.5 mL N/A
a
Dosage Level(s) 10-, 20-, 30-, 100-µg 10-, 20-, 30-µg N/A
Route of Administration Intramuscular injection Intramuscular injection Intramuscular injection
Use Experimental Experimental Placebo
IMP or NIMP IMP IMP IMP
Sourcing Provided centrally by the Provided centrally by the Provided centrally by the
sponsor sponsor sponsor
Packaging and Labeling Study intervention will be Study intervention will be Study intervention will be
provided in a glass vial as provided in a glass vial as provided in a glass or
open-label supply. Each open-label supply. Each plastic vial as open-label
vial will be labeled as vial will be labeled as supply. Each vial will be
required per country required per country labeled as required per
requirement requirement country requirement
a. Dependent upon safety and/or immunogenicity data generated during the course of this study, or the
BioNTech study conducted in Germany (BNT162-01), it is possible that groups may be started at the next
highest dose, groups may not be started, groups may be terminated early, and/or groups may be added
with dose levels below the lowest stated dose or intermediate between the lowest and highest stated doses.
The vaccine candidate selected for Phase 2/3 evaluation is BNT162b2 at a dose of 30 µg.
6.1.1. Manufacturing Process
The scale of the BNT162b2 manufacturing has been increased to support future supply.
BNT162b2 generated using the manufacturing process supporting an increased supply
(“Process 2”) will be administered to approximately 250 participants 16 to 55 years of age,
per lot, in the study. The safety and immunogenicity of prophylactic BNT162b2 in
individuals 16 to 55 years of age vaccinated with material generated using the existing
manufacturing process “Process 1,” and with material from lots generated using the
manufacturing process supporting increased supply, “Process 2,” will be described.
In brief, the process changes relate to the method of production for the DNA template that
RNA drug substance is transcribed from, and the RNA drug substance purification method.
The BNT162b2 drug product is then produced using a scaled-up LNP manufacturing process.
6.1.2. Administration
Participants will receive 1 dose of study intervention as randomized at each vaccination visit
(Visits 1 and 4 for Phase 1 participants, Visits 1 and 2 for Phase 2/3 participants) in
accordance with the study’s SoA. The volume to be administered may vary by vaccine
candidate and dose level; full details are described in the IP manual.
Page 46