Page 84 - Science
P. 84
RESEARCH | REPORT
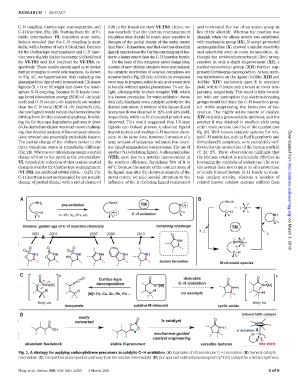
C–N coupling, Curtius-type rearrangement, and 0.39 in the transition state VI-TS3. Hence, we and envisioned the use of an amino group in
C–H insertion (Fig. 2B). Starting from the Ir(V)- can conclude that the Curtius rearrangement lieu of the alkoxide. Whereas the reaction was
imido intermediate VI, transition state calcu- transition stateshouldbemuchmoresensitive to sluggish when the alkoxy moiety was substituted
lations revealed that the C–N coupling is most changes of the partial charge of the metal center with tosylamide group (IX), N-acetyl–protected
facile, with a barrier of only 9.1 kcal/mol. Barriers than the C–H insertion, and that electron-donating aminoquinoline (X) showed a similar reactivity
for the Curtius-type rearrangement and C–Hinser- ligandsmayincreasetheCurtius-rearrangementbar- and selectivity even at room temperature. Al-
tion were slightly higher in energy: 9.6 kcal/mol rier to a larger extent than the C–Hinsertion barrier. though the tert-butyloxycarbonyl (Boc) group
for VI-TS2 and 12.0 kcal/mol for VI-TS3,re- On the basis of this computer-aided design idea, resulted in only a slight improvement (XI), a
spectively. These results encouraged us to devise a series of new iridium catalysts were synthesized; methyloxycarbonyl group (XII)further sup-
further strategies to avoid side reactions. As shown the catalytic reactivities of selected complexes are pressed Curtius-type decomposition. At last, meth-
in Fig. 2C, we hypothesized that replacing the summarized in Fig. 2D (24). All iridium complexes oxy substitution on the ligand (5-OMe: XIII and
phenylpyridine ligand with monoanionicLX-donor were easy to prepare, stable in air, and convenient 4-OMe: XIV) exclusively gave 5 in excellent
ligands (X = O or N) might shut down the inner- to handle without special precautions. To our de- yield within 6 hours and 2 hours at room tem-
sphere X–Ncoupling: BecauseN–N bonds [aver- light, alkoxypyridyl iridium complex VII,which perature, respectively. This result is fully consist-
age bond dissociation energy (BDE) of ~39 kcal/ is a known precatalyst for water-oxidation chem- ent with our assumption that electron-donating
mol] and O–N bonds (~50 kcal/mol) are weaker istry (25), displayed some catalytic activity for the groups would facilitate the C–H insertion prod-
than the C–N bond (BDE of ~73 kcal/mol) (23), desired amination: A mixture of the lactam 5 and uct while suppressing the formation of iso-
the new ligands would reduce the thermodynamic isocyanate 6 was obtained in 29% and 33% yield, cyanate. The highly active nature of catalyst
driving force for this undesired pathway. Inhibit- respectively, while no N–O coupled product was XIV enabled a gram-scalable synthesis, and the
ing the Curtius-type degradation pathway in favor observed. This result suggested that LX-type product 5 was obtained in excellent yield using
of the desired amidation was much more challeng- ligands can indeed prevent undesired ligand only 1 mole percent (mol %) of the catalyst (see
ing. Our detailed analysis of the computer simula- deconstruction and mediate C–H insertion chem- fig. S1). Well-known catalytic systems for rela-
tions revealed one potentially exploitable feature: istry. At the same time, however, formation of a ted C–H amidation, such as Ru(II)-porphyrin and
The partial charge of the iridium center in the large amount of isocyanate indicated that exten- dirhodium(II) complexes, were completely inef-
three transition states is remarkably different sive ligand manipulation was necessary. The use of fective for the production of the lactam scaffold Downloaded from
(Fig. 2B). Whereas our calculations assign a partial another N,O-chelating ligand, 8-alkoxyquinoline (7, 26, 27). These observations highlight that
charge of 0.40 to the metal in the intermediate (VIII), gave rise to a notable improvement in the iridium catalyst is particularly effective in
VI, remarkable reduction of that positive partial the reaction efficiency, furnishing 73% of 5 at leveraging the reactivity of acylnitrene. The pres-
charge is seen for the Curtius-type rearrangement 40°C. Because the nature of the contact atom of ent system does not require in situ protection
(VI-TS2, natural bond orbital NBO Ir =0.27).The the ligand may alter the electronic property of the of newly formed lactam N–H bonds to main-
C–H insertion is not accompanied by any notable metal center, we paid special attention to the tain catalyst activity, whereas a number of
change of partial charge, with a metal charge of influence of the X-chelating ligand component related known catalyst systems suffered from http://science.sciencemag.org/
on March 1, 2018
Fig. 1. A strategy for applying carbonylnitrene precursors in catalytic C–Hamidation. (A) Examples of intramolecular C–H amination. (B) General catalytic
mechanism. (C) Competitive decomposition pathway from the reactive intermediate. (D) Our approach with rational design of Cp*Ir(III) catalyst for g-lactam synthesis.
Hong et al., Science 359, 1016–1021 (2018) 2 March 2018 2of6