Page 91 - Cardiac Electrophysiology | A Modeling and Imaging Approach
P. 91
P. 91
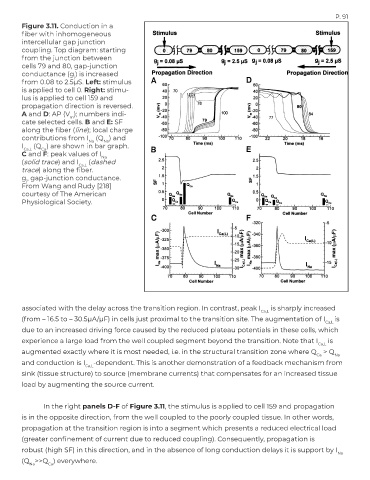
Figure 3.11. Conduction in a
fiber with inhomogeneous
intercellular gap junction
coupling. Top diagram: starting
from the junction between
cells 79 and 80, gap-junction
conductance (g ) is increased
j
from 0.08 to 2.5μS. Left: stimulus
is applied to cell 0. Right: stimu-
lus is applied to cell 159 and
propagation direction is reversed.
A and D: AP (V ); numbers indi-
m
cate selected cells. B and E: SF
along the fiber (line); local charge
contributions from I (Q ) and
Na
Na
I Ca,L (Q ) are shown in bar graph.
Ca
C and F: peak values of I Na
(solid trace) and I Ca,L (dashed
trace) along the fiber.
g , gap-junction conductance.
j
From Wang and Rudy [218]
courtesy of The American
Physiological Society.
associated with the delay across the transition region. In contrast, peak I is sharply increased
Ca,L
(from – 16.5 to – 30.5μA/μF) in cells just proximal to the transition site. The augmentation of I is
Ca,L
due to an increased driving force caused by the reduced plateau potentials in these cells, which
experience a large load from the well coupled segment beyond the transition. Note that I is
Ca,L
augmented exactly where it is most needed, i.e. in the structural transition zone where Q > Q
Ca Na
and conduction is I -dependent. This is another demonstration of a feedback mechanism from
Ca,L
sink (tissue structure) to source (membrane currents) that compensates for an increased tissue
load by augmenting the source current.
In the right panels D-F of Figure 3.11, the stimulus is applied to cell 159 and propagation
is in the opposite direction, from the well coupled to the poorly coupled tissue. In other words,
propagation at the transition region is into a segment which presents a reduced electrical load
(greater confinement of current due to reduced coupling). Consequently, propagation is
robust (high SF) in this direction, and in the absence of long conduction delays it is support by I
Na
(Q >>Q ) everywhere.
Na Ca