Page 984 - Clinical Small Animal Internal Medicine
P. 984
922 Section 9 Infectious Disease
VetBooks.ir (a) (b)
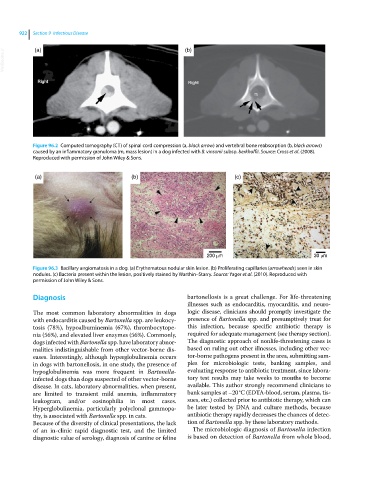
Figure 96.2 Computed tomography (CT) of spinal cord compression (a, black arrow) and vertebral bone reabsorption (b, black arrows)
caused by an inflammatory granuloma (m, mass lesion) in a dog infected with B. vinsonii subsp. berkhoffii. Source: Cross et al. (2008).
Reproduced with permission of John Wiley & Sons.
(a) (b) (c)
200 m 30 m
Figure 96.3 Bacillary angiomatosis in a dog. (a) Erythematous nodular skin lesion. (b) Proliferating capillaries (arrowheads) seen in skin
nodules. (c) Bacteria present within the lesion, positively stained by Warthin–Starry. Source: Yager et al. (2010). Reproduced with
permission of John Wiley & Sons.
Diagnosis bartonellosis is a great challenge. For life‐threatening
illnesses such as endocarditis, myocarditis, and neuro-
The most common laboratory abnormalities in dogs logic disease, clinicians should promptly investigate the
with endocarditis caused by Bartonella spp. are leukocy- presence of Bartonella spp. and presumptively treat for
tosis (78%), hypoalbuminemia (67%), thrombocytope- this infection, because specific antibiotic therapy is
nia (56%), and elevated liver enzymes (56%). Commonly, required for adequate management (see therapy section).
dogs infected with Bartonella spp. have laboratory abnor- The diagnostic approach of nonlife‐threatening cases is
malities indistinguishable from other vector‐borne dis- based on ruling out other illnesses, including other vec-
eases. Interestingly, although hyperglobulinemia occurs tor‐borne pathogens present in the area, submitting sam-
in dogs with bartonellosis, in one study, the presence of ples for microbiologic tests, banking samples, and
hypoglobulinemia was more frequent in Bartonella‐ evaluating response to antibiotic treatment, since labora-
infected dogs than dogs suspected of other vector‐borne tory test results may take weeks to months to become
disease. In cats, laboratory abnormalities, when present, available. This author strongly recommend clinicians to
are limited to transient mild anemia, inflammatory bank samples at –20 °C (EDTA‐blood, serum, plasma, tis-
leukogram, and/or eosinophilia in most cases. sues, etc.) collected prior to antibiotic therapy, which can
Hyperglobulinemia, particularly polyclonal gammopa- be later tested by DNA and culture methods, because
thy, is associated with Bartonella spp. in cats. antibiotic therapy rapidly decreases the chances of detec-
Because of the diversity of clinical presentations, the lack tion of Bartonella spp. by these laboratory methods.
of an in‐clinic rapid diagnostic test, and the limited The microbiologic diagnosis of Bartonella infection
diagnostic value of serology, diagnosis of canine or feline is based on detection of Bartonella from whole blood,