Page 220 - Clinical Small Animal Internal Medicine
P. 220
188 Section 3 Cardiovascular Disease
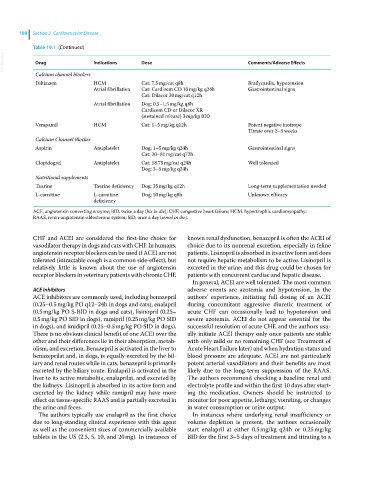
Table 19.1 (Continued)
VetBooks.ir Drug Indications Dose Comments/Adverse Effects
Calcium channel blockers
Diltiazem HCM Cat: 7.5 mg/cat q8h Bradycardia, hypotension
Atrial fibrillation Cat: Cardizem CD 10 mg/kg q24h Gastrointestinal signs
Cat: Dilacor 30 mg/cat q12h
Atrial fibrillation Dog: 0.5–1.5 mg/kg q8h
Cardizem CD or Dilacor XR
(sustained release) 3 mg/kg BID
Verapamil HCM Cat: 1–5 mg/kg q12h Potent negative inotrope
Titrate over 2–3 weeks
Calcium Channel Blocker
Aspirin Antiplatelet Dog: 1–5 mg/kg q24h Gastrointestinal signs
Cat: 20–81 mg/cat q72h
Clopidogrel Antiplatelet Cat: 18.75 mg/cat q24h Well tolerated
Dog: 3–5 mg/kg q24h
Nutritional supplements
Taurine Taurine deficiency Dog: 35 mg/kg q12h Long‐term supplementation needed
L‐carnitine L‐carnitine Dog: 50 mg/kg q8h Unknown efficacy
deficiency
ACE, angiotensin converting enzyme; BID, twice a day (bis in die); CHF, congestive heart failure; HCM, hypertrophic cardiomyopathy;
RAAS, renin‐angiotensin‐aldosterone system; SID, once a day (semel in die).
CHF and ACEI are considered the first‐line choice for known renal dysfunction, benazepril is often the ACEI of
vasodilator therapy in dogs and cats with CHF. In humans, choice due to its nonrenal excretion, especially in feline
angiotensin receptor blockers can be used if ACEI are not patients. Lisinopril is absorbed in its active form and does
tolerated (intractable cough is a common side‐effect), but not require hepatic metabolism to be active. Lisinopril is
relatively little is known about the use of angiotensin excreted in the urine, and this drug could be chosen for
receptor blockers in veterinary patients with chronic CHF. patients with concurrent cardiac and hepatic disease.
In general, ACEI are well tolerated. The most common
ACE Inhibitors adverse events are azotemia and hypotension. In the
ACE inhibitors are commonly used, including benazepril authors’ experience, initiating full dosing of an ACEI
(0.25–0.5 mg/kg PO q12–24h in dogs and cats), enalapril during concomitant aggressive diuretic treatment of
(0.5 mg/kg PO S‐BID in dogs and cats), lisinopril (0.25– acute CHF can occasionally lead to hypotension and
0.5 mg/kg PO SID in dogs), ramipril (0.25 mg/kg PO SID severe azotemia. ACEI do not appear essential for the
in dogs), and imidipril (0.25–0.5 mg/kg PO SID in dogs). successful resolution of acute CHF, and the authors usu-
There is no obvious clinical benefit of one ACEI over the ally initiate ACEI therapy only once patients are stable
other and their differences lie in their absorption, metab- with only mild or no remaining CHF (see Treatment of
olism, and excretion. Benazepril is activated in the liver to Acute Heart Failure later) and when hydration status and
benazeprilat and, in dogs, is equally excreted by the bil- blood pressure are adequate. ACEI are not particularly
iary and renal routes while in cats, benazepril is primarily potent arterial vasodilators and their benefits are most
excreted by the biliary route. Enalapril is activated in the likely due to the long‐term suppression of the RAAS.
liver to its active metabolite, enalaprilat, and excreted by The authors recommend checking a baseline renal and
the kidneys. Lisinopril is absorbed in its active form and electrolyte profile and within the first 10 days after start-
excreted by the kidney while ramipril may have more ing the medication. Owners should be instructed to
effect on tissue‐specific RAAS and is partially excreted in monitor for poor appetite, lethargy, vomiting, or changes
the urine and feces. in water consumption or urine output.
The authors typically use enalapril as the first choice In instances where underlying renal insufficiency or
due to long‐standing clinical experience with this agent volume depletion is present, the authors occasionally
as well as the convenient sizes of commercially available start enalapril at either 0.5 mg/kg q24h or 0.25 mg/kg
tablets in the US (2.5, 5, 10, and 20 mg). In instances of BID for the first 3–5 days of treatment and titrating to a