Page 82 - Heart Transplant Protocol
P. 82
Heart Function Service: Heart Transplant Protocols
Protocol for Administration of Valganciclovir (Valcyte®)
Description: Valganciclovir is a prodrug of ganciclovir and is rapidly converted to ganciclovir by
intestinal and hepatic esterases after oral administration. Ganciclovir is a synthetic analogue of
2’-deoxyguanosine and inhibits replication of cytomegalovirus.
Indication: approved for the prevention of CMV disease in kidney, heart, and kidney-pancreas
transplant patients and not indicated for use in either adult or pediatric liver transplant
patients. It has been used off-label for CMV/EBV treatment and prevention in solid organ
transplant patients, including liver patients.
Dose:
Children < 13 yo:
Treatment dose: 15 mg/kg po BID (max 900mg/dose)
Prophylactic dose: 15 mg/kg po QDAY (max 900mg/dose) for 9 months
Children ≥ 13 yo:
Treatment dose: 900mg po BID
Prophylactic dose: 900mg po QDAY for 9 months
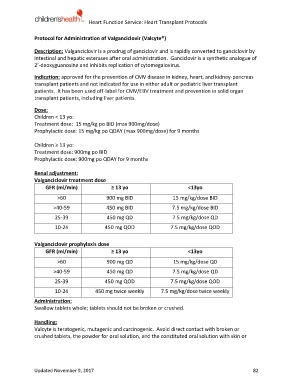
Renal adjustment:
Valganciclovir treatment dose
GFR (ml/min) ≥ 13 yo <13yo
>60 900 mg BID 15 mg/kg/dose BID
>40-59 450 mg BID 7.5 mg/kg/dose BID
25-39 450 mg QD 7.5 mg/kg/dose QD
10-24 450 mg QOD 7.5 mg/kg/dose QOD
Valganciclovir prophylaxis dose
GFR (ml/min) ≥ 13 yo <13yo
>60 900 mg QD 15 mg/kg/dose QD
>40-59 450 mg QD 7.5 mg/kg/dose QD
25-39 450 mg QOD 7.5 mg/kg/dose QOD
10-24 450 mg twice weekly 7.5 mg/kg/dose twice weekly
Administration:
Swallow tablets whole; tablets should not be broken or crushed.
Handling:
Valcyte is teratogenic, mutagenic and carcinogenic. Avoid direct contact with broken or
crushed tablets, the powder for oral solution, and the constituted oral solution with skin or
Updated November 9, 2017 82