Page 22 - Chemistry
P. 22
the four elements in order of their reactivity, starting with the most reactive.
h) Explain how hydrogen is used in the manufacture of margarine.
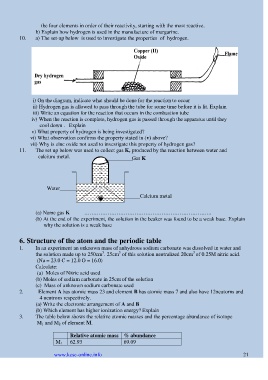
10. a) The set-up below is used to investigate the properties of hydrogen.
i) On the diagram, indicate what should be done for the reaction to occur
ii) Hydrogen gas is allowed to pass through the tube for some time before it is lit. Explain
iii) Write an equation for the reaction that occurs in the combustion tube
iv) When the reaction is complete, hydrogen gas is passed through the apparatus until they
cool down . Explain
v) What property of hydrogen is being investigated?
vi) What observation confirms the property stated in (v) above?
vii) Why is zinc oxide not used to investigate this property of hydrogen gas?
11. The set up below was used to collect gas K, produced by the reaction between water and
calcium metal. Gas K
o
o
o
o
Water o
o Calcium metal
o
(a) Name gas K ……………………………………………………………..
(b) At the end of the experiment, the solution in the beaker was found to be a weak base. Explain
why the solution is a weak base
6. Structure of the atom and the periodic table
1. In an experiment an unknown mass of anhydrous sodium carbonate was dissolved in water and
3
3
3
the solution made up to 250cm . 25cm of this solution neutralized 20cm of 0.25M nitric acid.
(Na = 23.0 C = 12.0 O = 16.0)
Calculate:
(a) Moles of Nitric acid used
(b) Moles of sodium carbonate in 25cm of the solution
(c) Mass of unknown sodium carbonate used
2. Element A has atomic mass 23 and element B has atomic mass 7 and also have 12neutorns and
4 neutrons respectively.
(a) Write the electronic arrangement of A and B
(b) Which element has higher ionization energy? Explain
3. The table below shows the relative atomic masses and the percentage abundance of isotope
M 1 and M 2 of element M.
Relative atomic mass % abundance
M 1 62.93 69.09
www.kcse-online.info 21