Page 58 - Chemistry
P. 58
(i) Identify solid E
(ii) Complete the diagram to show how the gas can be collected
(iii) Write an equation to show how the gas is formed
(iv) Complete the equation below: )
C 2H 2 + 2I 2
(v) What is the role of sand in the experiment?
(b) (i) Explain the meaning of esterification
(ii) Complete the equation below :
CH 3COOCH 3 + H 2O
(iii) What type of reaction is occurring above
(c) Given the reaction:
Solid F
C 8H 18 N + C 2H 4
(i) Identify substance:
F………………………………… N………………………………
(ii) Name the process represented above?
(d) Give one use of substance N
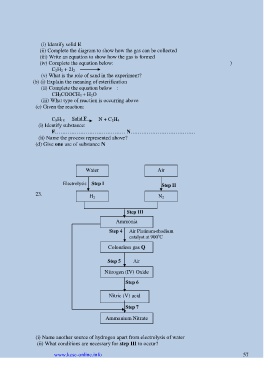
Water Air
Electrolysis Step I
Step II
23. H 2 N 2
Step III
Ammonia
Step 4 Air Platinum-rhodium
o
catalyst at 900 C
Colourless gas Q
Step 5 Air
Nitrogen (IV) Oxide
Step 6
Nitric (V) acid
Step 7
Ammonium Nitrate
(i) Name another source of hydrogen apart from electrolysis of water
(ii) What conditions are necessary for step III to occur?
www.kcse-online.info 57